Abstract
Purposes
This work aimed to assess the possible role of TRIM25 in regulating hyperglycemia-induced inflammation, senescence, and oxidative stress in retinal microvascular endothelial cells, all of which exert critical roles in the pathological process of diabetic retinopathy.
Methods
The effects of TRIM25 were investigated using streptozotocin-induced diabetic mice, human primary retinal microvascular endothelial cells cultured in high glucose, and adenoviruses for TRIM25 knockdown and overexpression. TRIM25 expression was evaluated by western blot and immunofluorescence staining. Inflammatory cytokines were detected by western blot and quantitative real-time PCR. Cellular senescence level was assessed by detecting senescent marker p21 and senescence‐associated‐β‐galactosidase activity. The oxidative stress state was accessed by detecting reactive oxygen species and mitochondrial superoxide dismutase.
Results
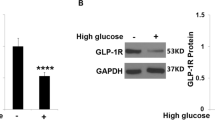
TRIM25 expression is elevated in the endothelial cells of the retinal fibrovascular membrane from diabetic patients compared with that of the macular epiretinal membrane from non-diabetic patients. Moreover, we have also observed a significant increase in TRIM25 expression in diabetic mouse retina and retinal microvascular endothelial cells under hyperglycemia. TRIM25 knockdown suppressed hyperglycemia-induced inflammation, senescence, and oxidative stress in human primary retinal microvascular endothelial cells while TRIM25 overexpression further aggregates those injuries. Further investigation revealed that TRIM25 promoted the inflammatory responses mediated by the TNF-α/NF-κB pathway and TRIM25 knockdown improved cellular senescence by increasing SIRT3. However, TRIM25 knockdown alleviated the oxidative stress independent of both SIRT3 and mitochondrial biogenesis.
Conclusion
Our study proposed TRIM25 as a potential therapeutic target for the protection of microvascular function during the progression of diabetic retinopathy.





Similar content being viewed by others
References
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239. https://doi.org/10.1056/NEJMra1005073
Teo ZL, Tham YC, Yu M et al (2021) Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128(11):1580–1591. https://doi.org/10.1016/j.ophtha.2021.04.027
Hammes HP (2018) Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia 61(1):29–38. https://doi.org/10.1007/s00125-017-4435-8
Baker RG, Hayden MS, Ghosh S (2011) NF-κB, inflammation, and metabolic disease. Cell Metab 13(1):11–22. https://doi.org/10.1016/j.cmet.2010.12.008
Dammak A, Huete-Toral F, Carpena-Torres C, et al. (2021) From oxidative stress to inflammation in the posterior ocular diseases: diagnosis and treatment. Pharmaceutics 13(9). https://doi.org/10.3390/pharmaceutics13091376
Di Micco R, Krizhanovsky V, Baker D et al (2021) Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 22(2):75–95. https://doi.org/10.1038/s41580-020-00314-w
Sabbatinelli J, Prattichizzo F, Olivieri F et al (2019) Where metabolism meets senescence: focus on endothelial cells. Front Physiol 10:1523. https://doi.org/10.3389/fphys.2019.01523
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070. https://doi.org/10.1161/circresaha.110.223545
Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007:43603. https://doi.org/10.1155/2007/43603
Heikel G, Choudhury NR, Michlewski G (2016) The role of Trim25 in development, disease and RNA metabolism. Biochem Soc Trans 44(4):1045–1050. https://doi.org/10.1042/bst20160077
Lee JM, Choi SS, Lee YH et al (2018) The E3 ubiquitin ligase TRIM25 regulates adipocyte differentiation via proteasome-mediated degradation of PPARγ. Exp Mol Med 50(10):1–11. https://doi.org/10.1038/s12276-018-0162-6
Li C, Dou P, Lu X, et al. (2022) Identification and validation of TRIM25 as a glucose metabolism regulator in prostate cancer. International journal of molecular sciences 23(16). https://doi.org/10.3390/ijms23169325
Wan T, Li X, Li Y (2021) The role of TRIM family proteins in autophagy, pyroptosis, and diabetes mellitus. Cell Biol Int 45(5):913–926. https://doi.org/10.1002/cbin.11550
Liu Y, Liu K, Huang Y et al (2020) TRIM25 promotes TNF-α-induced NF-κB activation through potentiating the K63-linked ubiquitination of TRAF2. J Immunol (Baltimore, Md : 1950) 204(6):1499–1507. https://doi.org/10.4049/jimmunol.1900482
Park HS, Lu Y, Pandey K et al (2021) NLRP3 inflammasome activation enhanced by TRIM25 is targeted by the NS1 protein of 2009 pandemic influenza a virus. Front Microbiol 12:778950. https://doi.org/10.3389/fmicb.2021.778950
Crespo-Garcia S, Tsuruda PR, Dejda A et al (2021) Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab 33(4):818-832.e817. https://doi.org/10.1016/j.cmet.2021.01.011
Kida Y, Goligorsky MS (2016) Sirtuins, cell senescence, and vascular aging. Can J Cardiol 32(5):634–641. https://doi.org/10.1016/j.cjca.2015.11.022
Chen T, Ma C, Fan G et al (2021) SIRT3 protects endothelial cells from high glucose-induced senescence and dysfunction via the p53 pathway. Life Sci 264:118724. https://doi.org/10.1016/j.lfs.2020.118724
Tang X, Luo YX, Chen HZ et al (2014) Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 5:175. https://doi.org/10.3389/fphys.2014.00175
Han L, Li J, Li J et al (2020) Activation of AMPK/Sirt3 pathway by phloretin reduces mitochondrial ROS in vascular endothelium by increasing the activity of MnSOD via deacetylation. Food Funct 11(4):3073–3083. https://doi.org/10.1039/c9fo02334h
Chen ML, Zhu XH, Ran L, et al. (2017) Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc 6(9). https://doi.org/10.1161/jaha.117.006347
Bagul PK, Katare PB, Bugga P, et al. (2018) SIRT-3 modulation by resveratrol improves mitochondrial oxidative phosphorylation in diabetic heart through deacetylation of TFAM. Cells 7(12). https://doi.org/10.3390/cells7120235
Lee NR, Kim HI, Choi MS et al (2015) Regulation of MDA5-MAVS antiviral signaling axis by TRIM25 through TRAF6-mediated NF-κB activation. Mol Cells 38(9):759–764. https://doi.org/10.14348/molcells.2015.0047
Mei P, Xie F, Pan J et al (2021) E3 ligase TRIM25 ubiquitinates RIP3 to inhibit TNF induced cell necrosis. Cell Death Differ 28(10):2888–2899. https://doi.org/10.1038/s41418-021-00790-3
Tian X, Xue Y, Xie G et al (2021) (-)-Epicatechin ameliorates cigarette smoke-induced lung inflammation via inhibiting ROS/NLRP3 inflammasome pathway in rats with COPD. Toxicol Appl Pharmacol 429:115674. https://doi.org/10.1016/j.taap.2021.115674
Ge MX, Shi YK, Liu D (2022) Tripartite motif-containing 25 facilitates immunosuppression and inhibits apoptosis of glioma via activating NF-κB. Exp Biol Med (Maywood) 247(17):1529–1541. https://doi.org/10.1177/15353702221099460
Rufini A, Tucci P, Celardo I et al (2013) Senescence and aging: the critical roles of p53. Oncogene 32(43):5129–5143. https://doi.org/10.1038/onc.2012.640
Vigneron A, Vousden KH (2010) p53, ROS and senescence in the control of aging. Aging 2(8):471–474. https://doi.org/10.18632/aging.100189
Zhang P, Elabd S, Hammer S et al (2015) TRIM25 has a dual function in the p53/Mdm2 circuit. Oncogene 34(46):5729–5738. https://doi.org/10.1038/onc.2015.21
Chen Y, Zhao Y, Yang X et al (2022) USP44 regulates irradiation-induced DNA double-strand break repair and suppresses tumorigenesis in nasopharyngeal carcinoma. Nat Commun 13(1):501. https://doi.org/10.1038/s41467-022-28158-2
Nishikawa T, Edelstein D, Du XL et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790. https://doi.org/10.1038/35008121
Mao X, Ji M, Kang L et al (2022) XRCC5 downregulated by TRIM25 is susceptible for lens epithelial cell apoptosis. Cell Signal 94:110314. https://doi.org/10.1016/j.cellsig.2022.110314
Yang X, Zhang F, Liu X et al (2022) FOXO4 mediates resistance to oxidative stress in lens epithelial cells by modulating the TRIM25/Nrf2 signaling. Exp Cell Res 420(1):113340. https://doi.org/10.1016/j.yexcr.2022.113340
Liu Y, Tao S, Liao L et al (2020) TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun 11(1):348. https://doi.org/10.1038/s41467-019-14190-2
Funding
This work was supported by the Science and Technology Research Project of Songjiang District [grant number 2020SJ300]; the National Key R&D Program of China [grant numbers 2016YFC0904800, 2019YFC0840607]; National Science and Technology Major Project of China [grant number 2017ZX09304010]; and Shanghai Key Clinical Specialty.
Author information
Authors and Affiliations
Contributions
DS, NW, and FW designed this study. DS and SL executed the experiments. NW, SC, and YS collected the human specimens, carried out experiments, and analyzed data. DS and SZ maintained the mice. NW, FW, and QG provided resources, supervised the experiments, and participated in the data analyses. DS, FW, and NW wrote the article with input from all authors. All authors proofread the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Animals were treated in accordance with the ARRIVE guidelines and the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the Ethics committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (reference number: 2019-A049-01).
Human samples were collected with informed consent following the guidelines of the Helsinki Declaration. This study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (reference number: 2020SQ100).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, D., Li, S., Chen, S. et al. TRIM25 inhibition attenuates inflammation, senescence, and oxidative stress in microvascular endothelial cells induced by hyperglycemia. Graefes Arch Clin Exp Ophthalmol 262, 81–91 (2024). https://doi.org/10.1007/s00417-023-06160-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06160-8