Abstract
Purpose
To determine the characteristics of eyes diagnosed with Best vitelliform macular dystrophy (BVMD) and autosomal recessive bestrophinopathy (ARB) complicated by choroidal neovascularization (CNV).
Methods
This was a retrospective, multicenter observational case series. Fourteen genetically confirmed BVMD patients and 9 ARB patients who had been examined in 2 ophthalmological institutions in Japan were studied. The findings in a series of ophthalmic examinations including B-scan optical coherence tomography (OCT) and OCT angiography (OCTA) were reviewed.
Results
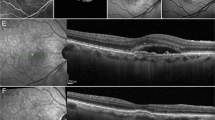
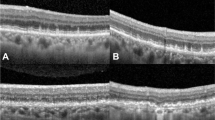
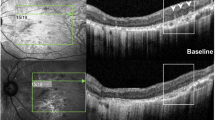
CNV was identified in 5 eyes (17.9%) of BVMD patients and in 2 eyes (11.1%) of ARB patients. Three of 5 eyes with BVMD were classified as being at the vitelliruptive stage and 2 eyes at the atrophic stage. The CNV in 2 BVMD eyes were diagnosed as exudative because of acute visual acuity reduction, retinal hemorrhage, and intraretinal fluid, while the CNV in 3 BVMD eyes and 2 ARB eyes were diagnosed as non-exudative. The visual acuity of the two eyes with exudative CNV did not improve despite anti-VEGF treatments. None of the eyes with non-exudative CNV had a reduction of their visual acuity for at least 4 years. All of the CNV were located within hyperreflective materials which were detected in 16 eyes (57.1%) of the BVMD eyes and in 7 eyes (38.9%) of the ARB eyes.
Conclusions
CNV is a relatively common complication in BEST1-related retinopathy in Asian population as well. The prognosis of eyes with exudative CNV is not always good, and OCTA can detect CNV in eyes possessing hyperreflective materials.






Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author, SU, upon reasonable request.
References
Petrukhin K, Koisti MJ, Bakall B et al (1998) Identification of the gene responsible for Best macular dystrophy. Nat Genet 19:241–247. https://doi.org/10.1038/915
Johnson AA, Guziewicz KE, Lee CJ, Kalathur RC, Pulido JS, Marmorstein LY, Marmorstein AD (2017) Bestrophin 1 and retinal disease. Prog Retin Eye Res 58:45–69. https://doi.org/10.1016/j.preteyeres.2017.01.006
Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K (2000) Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A 97:12758–12763. https://doi.org/10.1073/pnas.220402097
Johnson AA, Lee YS, Chadburn AJ, Tammaro P, Manson FD, Marmorstein LY, Marmorstein AD (2014) Disease-causing mutations associated with four bestrophinopathies exhibit disparate effects on the localization, but not the oligomerization, of Bestrophin-1. Exp Eye Res 121:74–85. https://doi.org/10.1016/j.exer.2014.02.006
Best F II (1905) Über eine hereditäre maculaaffektion. Beitrag zur Vererbungslehre Z Augenheilk 13:199–212
Gass JDM (1997) Heredodystrophic disorders affecting the pigment epithelium and retina. In: Gass JD (ed) Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. St. Louis, C.V. Mosby, pp 304–325
Schatz P, Klar J, Andréasson S, Ponjavic V, Dahl N (2006) Variant phenotype of Best vitelliform macular dystrophy associated with compound heterozygous mutations in VMD2. Ophthalmic Genet 27:51–56. https://doi.org/10.1080/13816810600677990
Burgess R, Millar ID, Leroy BP et al (2008) Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet 82:19–31. https://doi.org/10.1016/j.ajhg.2007.08.004
Boon CJ, Klevering BJ, Leroy BJ, Hoyng CB, Keunen JE, den Hollander AI (2009) The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res 28:187–205. https://doi.org/10.1016/j.preteyeres.2009.04.002
Nakanishi A, Ueno S, Hayashi T, Katagiri S, Kominami T, Ito Y, Terasaki H et al (2016) Clinical and genetic findings of Autosomal recessive bestrophinopathy in Japanese cohort. Am J Ophthalmol 168:86–94. https://doi.org/10.1016/j.ajo.2016.04.023
Nakanishi A, Ueno S, Hayashi T et al (2020) Changes of cone photoreceptor mosaic in Autosomal recessive bestrophinopathy. Retina 40:181–186. https://doi.org/10.1097/IAE.0000000000002363
Hussain RN, Shahid FL, Empeslidis T, Ch’ng SW (2015) Use of intravitreal bevacizumab in a 9-year-old child with choroidal neovascularization associated with Autosomal recessive bestrophinopathy. Ophthalmic Genet 36:265–269. https://doi.org/10.3109/13816810.2014.962706
Khan KN, Mahroo OA, Islam F, Webster AR, Moore AT, Michaelides M (2017) Functional and anatomical outcomes of choroidal neovascularization complicating BEST1-related retinopathy. Retina 37:1360–1370. https://doi.org/10.1097/IAE.0000000000001357
Shahzad R, Siddiqui MA (2017) Choroidal neovascularization secondary to Best vitelliform macular dystrophy detected by optical coherence tomography angiography. J AAPOS 21:68–70. https://doi.org/10.1016/j.jaapos.2016.08.018
Cennamo G, Cesarano I, Vecchio EC, Reibaldi M, de Crecchio G (2012) Functional and anatomic changes in bilateral choroidal neovascularization associated with vitelliform macular dystrophy after intravitreal bevacizumab. J Ocul Pharmacol Ther 28:643–646
Chhablani J, Jalali S (2012) Intravitreal bevacizumab for choroidal neovascularization secondary to Best vitelliform macular dystrophy in a 6-year-old child. Eur J Ophthalmol 22:677–679. https://doi.org/10.5301/ejo.5000095
Guduru A, Gupta A, Tyagi M, Jalali S, Chhablani J (2018) Optical coherence tomography angiography characterisation of Best disease and associated choroidal neovascularisation. Br J Ophthalmol 102:444–447. https://doi.org/10.1136/bjophthalmol-2017-310586
Parodi MB, Romano F, Cicinelli MV, Rabiolo A, Arrigo A, Pierro L, Iacono P, Bandello F (2018) Retinal vascular impairment in Best vitelliform macular dystrophy assessed by means of optical coherence tomography angiography. Am J Ophthalmol 187:61–70. https://doi.org/10.1016/j.ajo.2017.12.013
Parodi MB, Arrigo A, Bandello F (2020) Optical coherence tomography angiography quantitative assessment of macular neovascularization in Best vitelliform macular dystrophy. Invest Ophthalmol Vis Sci 61:61. https://doi.org/10.1167/iovs.61.6.6
Kumar V, Chatra K (2018) Fibrotic pillar leads to focal choroidal excavation in Best vitelliform dystrophy. Graefes Arch Clin Exp Ophthalmol 256:2083–2087. https://doi.org/10.1007/s00417-018-4120-8
Grossniklaus HE, Green WR (2004) Choroidal neovascularization. Am J Ophthalmol 137:496–503. https://doi.org/10.1016/j.ajo.2003.09.042
Fujiki R, Ikeda M, Yoshida A et al (2018) Assessing the accuracy of variant detection in cost-effective gene panel testing by next-generation sequencing. J Mol Diagn 20:572–582. https://doi.org/10.1016/j.jmoldx.2018.04.004
Mizobuchi K, Hayashi T, Yoshitake K, Fujinami K, Tachibana T, Tsunoda K, Iwata T, Nakano T (2020) Novel homozygous CLN3 missense variant in isolated retinal dystrophy: a case report and electron microscopic findings. Mol Genet Genomic Med 8:e1308. https://doi.org/10.1002/mgg3.1308
Katagiri S, Hayashi T, Ohkuma Y, Sekiryu T, Takeuchi T, Gekka T, Kondo M, Iwata T, Tsuneoka H (2015) Mutation analysis of BEST1 in Japanese patients with Best’s vitelliform macular dystrophy. Br J Ophthalmol 99:1577–1582. https://doi.org/10.1136/bjophthalmol-2015-306830
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–423. https://doi.org/10.1038/gim.2015.30
Gao T, Tian C, Hu Q, Liu Z, Zou J, Huang L, Zhao M (2018) Clinical and mutation analysis of patients with Best vitelliform macular dystrophy or Autosomal recessive bestrophinopathy in Chinese population. Biomed Res Int 2018:4582816. https://doi.org/10.1155/2018/4582816
Acknowledgements
The authors thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute for the discussions and editing the final version of the manuscript. The swept-source optical coherence tomography device used in this study was on loan from Carl Zeiss Meditec.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant numbers 19K09928 to S.U. and 21K0975 to T.H. and Takayanagi Retina Research Award to M.M.
Author information
Authors and Affiliations
Contributions
MM and SU conceived of the presented idea. MM, KM, and SU collected clinical and image data. TH and YK performed gene analysis. MM, JT, and SU verified the analytical methods. YI, HT, and KN helped supervise the project. SU supervised the project. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The procedures used in this study adhered to the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board (IRB) of Nagoya University (approval number 2020–0173) and Jikei University School of Medicine (approval number 24–231, 6997).
Consent to participate
Written informed consents were obtained from all the patient.
Consent for publication
I, SU, transfers to Springer the publication rights and he warrants that his contribution is original and that he has full power to make this grant. The author signs for and accepts responsibility for releasing this material on behalf of any and all co-authors.
I understand that the text and any pictures published in the article will be used only in educational publications intended for professional or if the publication or product is published on an open access basis, I understand that it will be freely available on the internet and may be seen by the general public. The pictures and text may also appear on other websites or in print, and may be translated into other languages or used for commercial purposes. I understand that the information will be published without my name attached, but that full anonymity cannot be guaranteed. I have been offered the opportunity to read the manuscript. I acknowledge that it is not possible to ensure complete anonymity, and someone may be able to recognize me. However, by signing this consent form, I do not in any way give up, waive, or remove my rights to privacy. I may revoke my consent at any time before publication, but once the information has been committed to publication (“gone to press”), revocation of the consent is no longer possible.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyagi, M., Takeuchi, J., Koyanagi, Y. et al. Clinical findings in eyes with BEST1-related retinopathy complicated by choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 260, 1125–1137 (2022). https://doi.org/10.1007/s00417-021-05447-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05447-y