Abstract
Purpose
To investigate the optical coherence tomography (OCT) en face reconstruction of the choroid in different phenotypes of non-neovascular age-related macular degeneration (AMD), to identify the relative distribution of the vascular patterns of the Haller’s layer in each AMD category.
Methods
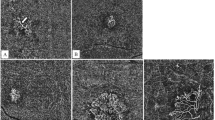
Retrospective study enrolling consecutive patients with non-neovascular AMD. Patients were divided into the following: (1) those with reticular pseudodrusen (RPD); (2) those with small (< 63 μm) or medium–large drusen (63–124 μm); (3) those with geographic atrophy (GA). Qualitative analysis of the en face images provided by CIRRUS HD-OCT 5000 (Carl Zeiss Meditech, Inc., Dublin, USA) was performed, identifying five arrangements of Haller’s vessels: temporal herringbone, branched from below, laterally diagonal, double arcuate, and reticular. Choroidal thickness (CT) was measured from structural OCT. Healthy age-matched subjects were included as a control group.
Results
Fifty-eight eyes of 58 patients (20 eyes with RPD; 22 eyes with drusen; 16 eyes with GA) and 18 control eyes were enrolled. The laterally diagonal configuration was the most prevalent (40.0%) in the RPD group; the reticular pattern was the most frequent in the drusen group (50.0%); the double arcuate (62.5%) was the most recurrent pattern in patients with GA. In the control group, the temporal herringbone (38.9%) arrangement was the most represented. The CT associated with the temporal herringbone and reticular arrangement was significantly higher compared to the branched from below (p < 0.001), the laterally diagonal (p = 0.014), and the double arcuate pattern (p = 0.009).
Conclusion
Different phenotypes of non-neovascular AMD present a specific distribution of vascular arrangement on en face OCT. The temporal herringbone and the reticular pattern (the ones more associated in a physiological setting) disclosed a thicker choroid compared to the arrangements more represented in non-neovascular AMD-correlated phenotypes.




Similar content being viewed by others
References
Klein R, Klein BE, Linton KL (1992) Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 99:933–943
Bandello F, Sacconi R, Querques L, Corbelli E, Cicinelli MV, Querques G (2017) Recent advances in the management of dry age-related macular degeneration: A review. F1000Res 6:245
Rabiolo A, Sacconi R, Cicinelli MV, Querques L, Bandello F, Querques G (2017) Spotlight on reticular pseudodrusen. Clin Ophthalmol 11:1707–1718
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 50:4982–4991
Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA (2009) Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 116:488–96 e482
Sacconi R, Corbelli E, Carnevali A, Querques L, Bandello F, Querques G (2018) Optical coherence tomography angiography in geographic atrophy. Retina. 38:2350–2355
Corbelli E, Sacconi R, Rabiolo A et al (2018) Optical coherence tomography angiography in the evaluation of geographic atrophy area extension [published correction appears in Invest Ophthalmol Vis Sci. 2017. 1;59:1801]. Invest Ophthalmol Vis Sci 58:5201–5208
Savastano MC, Rispoli M, Savastano A, Lumbroso B (2015) En face optical coherence tomography for visualization of the choroid. Ophthalmic Surg Lasers Imaging Retina 46:561–565
Savastano MC, Dansingani KK, Rispoli M et al (2018) Classification of Haller vessel arrangements in acute and chronic central serous chorioretinopathy imaged with en face optical coherence tomography. Retina. 38:1211–1215
Sadda SR, Guymer R, Holz FG et al (2018) Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 125:537–548
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500
Zhao J, Wang YX, Zhang Q, Wei WB, Xu L, Jonas JB (2018) Macular choroidal small-vessel layer, Sattler’s layer and Haller’s layer thicknesses: The Beijing Eye Study. Sci Rep 8:4411
Usui S, Ikuno Y, Akiba M et al (2012) Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci 53:2300–2307
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282
Seddon JM, McLeod DS, Bhutto IA et al (2016) Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol 134:1272–1280
Dansingani KK, Balaratnasingam C, Naysan J, Freund KB (2016) En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 36:499–516
Querques G, Lattanzio R, Querques L et al (2012) Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci 53:6017–6024
Ferrara D, Waheed NK, Duker JS (2016) Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res 52:130–155
Cicinelli MV, Rabiolo A, Marchese A et al (2017) Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol 101:1193–1200
Zheng F, Gregori G, Schaal KB et al (2016) Choroidal thickness and choroidal vessel density in nonexudative age-related macular degeneration using swept-source optical coherence tomography imaging. Invest Ophthalmol Vis Sci 57:6256–6264
Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT (1994) Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci 35:2857–2864
Sohn EH, Khanna A, Tucker BA, Abramoff MD, Stone EM, Mullins RF (2014) Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci 55:1352–1360
Corvi F, Souied EH, Capuano V et al (2017) Choroidal structure in eyes with drusen and reticular pseudodrusen determined by binarisation of optical coherence tomographic images. Br J Ophthalmol 101:348–352
Querques G, Querques L, Martinelli D et al (2011) Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 31:518–526
De Bats F, Wolff B, Mauget-Faysse M, Meunier I, Denis P, Kodjikian L (2013) Association of reticular pseudodrusen and early onset drusen. ISRN Ophthalmol 2013:273085
Zweifel SA, Imamura Y, Freund KB, Spaide RF (2011) Multimodal fundus imaging of pseudoxanthoma elasticum. Retina. 31:482–491
Wilde C, Lakshmanan A, Patel M, Morales MU, Dhar-Munshi S, Amoaku WM (2016) Prevalence of reticular pseudodrusen in newly presenting adult onset foveomacular vitelliform dystrophy. Eye (Lond) 30:817–824
Tan KA, Gupta P, Agarwal A et al (2016) State of science: Choroidal thickness and systemic health. Surv Ophthalmol 61:566–581
Acknowledgments
The authors thank Karl Anders Knutsson (IRCCS Ospedale San Raffaele, University Vita-Salute San Raffaele) for the English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Riccardo Sacconi, Maria Vittoria Cicinelli, Enrico Borrelli, Maria Cristina Savastano, Marco Rispoli, Bruno Lumbroso, Eleonora Corbelli, and Marco Casaluci certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Francesco Bandello is a consultant for Alcon (Fort Worth, TX, USA), Alimera Sciences (Alpharetta, GA, USA), Allergan Inc. (Irvine, CA, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, NY, USA), Genentech (San Francisco, CA, USA), Hoffmann-La-Roche (Basel, Switzerland), Novagali Pharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA).
Giuseppe Querques is a consultant for Alimera Sciences (Alpharetta, GA, USA), Allergan Inc. (Irvine, CA, USA), Amgen (Thousand Oaks, USA), Bayer Shering-Pharma (Berlin, Germany), Heidelberg (Germany), KBH (Chengdu; China), LEH Pharma (London, UK), Lumithera (Poulsbo; USA), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Sooft-Fidea (Abano, Italy), Zeiss (Dublin, USA).
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the (place name of institution and/or national research committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sacconi, R., Cicinelli, M.V., Borrelli, E. et al. Haller’s vessels patterns in non-neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 258, 2163–2171 (2020). https://doi.org/10.1007/s00417-020-04769-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04769-7