Abstract
Background
We aimed to compare the effects of intraperitoneal ghrelin and tacrolimus on vitreous levels of tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) in an experimental autoimmune uveitis model.
Methods
Twenty-four male rats, each weighing 300 g, were assigned into four groups, six rats in each. All the rats, except for those in group 1, were injected intravitreally with concanavalin a to induce experimental uveitis. The development of uveitis was confirmed by the histopathologic examination of two rat globes from each group. The rats in group 2 were not given any treatment after uveitis was induced. The rats in group 3 were administered 1 mg/kg/day of intraperitoneal tacrolimus on days 0, 1, 3, 5 and 7 following the induction of uveitis (on the 14th day of study). The rats in group 4 were given 10 ng/kg/day of intraperitoneal ghrelin for 7 days following the induction of uveitis. On the 21st day of the study, all rats were sacrificed, and the eyes enucleated were subjected to histopathologic examination. Vitreous levels of TNF-α, IL-1 and IL-6 were measured by the enzyme-linked immunosorbent assay method.
Results
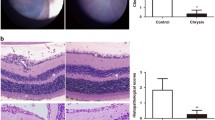
The histopathologic evaluation carried out to confirm the development of uveitis revealed destruction in the retinae and ciliary bodies of the immunized rats. The mean vitreous levels of TNF-α, IL-1 and IL-6 were significantly higher in the sham group than in the control group (p < 0.05). The levels of these three cytokines showed a significant decrease in the tacrolimus treatment group (p < 0.05). Cytokine levels decreased in the ghrelin treatment group relative to the control group; however, the decrease was not found statistically significant (p > 0.05).
Conclusions
Tacrolimus could be effective in uveitis treatment by neutralizing or decreasing the levels of cytokines such as TNF-α, IL-1 and IL-6 that have a critical part in the pathogenesis of uveitis. However, ghrelin failed to produce the desired effect. Further studies using different doses and different ways of administration are needed to determine the effective dose of ghrelin in uveitis.

Similar content being viewed by others
References
Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chignell AH, Dumonde DC (1992) Cytokines in human intraocular inflammation. Curr Eye Res 11:187–191
de Vos AF, Hoekzema R, Kijlstra A (1992) Cytokines and uveitis: a review. Curr Eye Res 11:581–597
de Vos AF, van Haren MA, Verhagen C, Hoekzema R, Kijlstra A (1994) Kinetics of intraocular tumor necrosis factor and interleukin- 6 in endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci 35:1100–1106
Bierly JR, Nozik RA (1992) Management of uveitis. Curr Opin Ophthalmol 3:527–533
Gordon DM (1956) Prednisone and prednisolone in ocular disease. Am J Ophthalmol 41:593–600
Foeldvari I, Wierk A (2005) Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol 32:362–365
Foster CS (2003) Diagnosis and treatment of juvenile idiopathic arthritis-associated uveitis. Curr Opin Ophthalmol 14:395–398
Lustig MJ, Cunningham ET (2003) Use of immunosuppressive agents in uveitis. Curr Opin Ophthalmol 14:399–412
Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED (2006) Risk of new-onset uveitis in patients with juvenile idiopathic arthritis treated with anti-TNFα agents. J Pediatr 149:833–836
Tynjälä P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K (2007) Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis 66:548–550
Miyata S, Ohkuba Y, Mutoh S (2005) A review of the action of tacrolimus (FK506) on experimental models of rheumatoid arthritis. Inflamm Res 54:1–9
Morikawa K, Oseko F, Morikowa S (1992) The distinct effects of FK506 on the activation, proliferation, and differentiation of human B lymphocytes. Transplantation 54:1025–1030
Sengoku T, Kishi S, Sakuma S, Ohkubo Y, Goto T (2000) FK506 inhibition of histamine release and cytokine production by mast cells and basophils. Int J Immunopharmacol 22:189–201
Sasakawa T, Sasakawa Y, Ohkubo Y, Mutoh S (2005) FK506 inhibits prostaglandin E2 production from synovial cells by suppressing peripheral blood mononuclear cells. Int Immunopharmacol 5:1291–1297
Sakuma S, Kato Y, Nishigaki F, Sasakawa T, Magari K, Miyata S, Ohkubo Y, Goto T (2000) FK506 potently inhibits T cell activation induced TNF-alpha and IL-1beta production in vitro by human peripheral blood mononuclear cells. Br J Pharmacol 130:1655–1663
Sakuma S, Kato Y, Nishigaki F, Magari K, Miyata S, Ohkubo Y, Goto T (2001) Effects of FK506 and other immunosuppressive anti-rheumatic agents on T cell activation mediated IL-6 and IgM production in vitro. Int Immunopharmacol 1:749–757
Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K, Yamamoto K (1999) A potent immunosuppressant FK506 inhibits IL-8 expression in human eosinophils. Mol Cell Biol Res Commun 1:72–77
Kilmartin DJ, Forrester JV, Dick AD (1998) Tacrolimus (FK506) in failed cyclosporin a therapy in endogenous posterior uveitis. Ocul Immunol Inflamm 6:101–109
Mochizuki M, Masuda K, Sakane T, Ito K, Kogure M, Sugino N, Usui M, Mizushima Y, Ohno S, Inaba G (1993) A clinical trial of FK506 in refractory uveitis. Am J Ophthalmol 115:763–769
Ishioka M, Ohno S, Nakamura S, Isobe K, Watanabe N, Ishigatsubo Y, Tanaka S (1994) FK506 treatment of noninfectious uveitis. Am J Ophthalmol 118:723–729
Murphy CC, Greiner K, Plskova J, Duncan L, Frost NA, Forrester JV, Dick AD (2005) Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol 123:634–641
Kojima M, Hosoda H, Date Y (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85:495–522
Aydin S, Ozkan Y, Caylak E, Aydin S (2006) Ghrelin and its biochemical functions. Turk Klin J Med Sci 26:272–283
Wren AM, Seal LJ, Cohen MA, Frost GS (2001) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992–5994
Tschop M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407:908–913
Dixit VD, Pyle RS, Schaffer EM, Collins GD (2004) Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 114:57–66
Dembinski A, Konturek SJ, Ceranowicz P (2003) Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol 54:561–573
Mathurin P, Gonzalez F, Kerdraon O, Leteurte E, Louvet A (2006) The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology 130:1617–1624
Chen J, Liu X, Shu Q, Li S (2008) Ghrelin attenuates lipopolysaccharide-induced acute lung Injury through NO pathway. Med Sci Monit 14:141–146
Granado M, Priego T, Martin AI (2005) Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab 288:486–492
Lightman S (2001) Uveitis: what do we know and how does it help? Clin Exp Ophthalmol 29:48–51
Er H, Uzmez E, Doğan N, Cumhurcu T (1999) The anti-inflammatory effects of nitro L arginine (L-NAME) and steroid in concanavalin A-ınduced uveitis. J Med Sci 29:233–239
Gwon A, Mantras C, Gruber L, Cunanan C (1993) Concanavalin A-induced posterior subcapsular cataract: a new model of cataractogenesis. Invest Ophthalmol Vis Sci 34:3483–3488
Sun M, Yang Y, Yang P, Lei B, Du L, Kijlstra A (2011) Regulatory effects of IFN-β on the development of experimental autoimmune uveoretinitis in B10RIII mice. PLoS One 6:e19870
Kawashima H, Fujino Y, Mochizuki M (1990) Antigen-specific suppressor cells induced by FK506 in experimental autoimmune uveoretinitis in the rat. Invest Ophthalmol Vis Sci 31:2500–2507
Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR (2008) Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med 205:799–810
Abbas AK, Pober JS (1997) Cytokines. Cellular and molecular immunology, 3rd edn. WB. Saunders Company, Pennsylvania, pp 1–48
Murray PI, Van Haren HR (1990) MAC: Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci 31:917–920
Rutanen EM (1993) Cytokines in reproduction. Ann Med 25:343–347
Galley HF, Webster NR (1996) The immuno-inflammatory cascade. Br J Anaesth 77:11–16
Hoekzema R, Murray PI, Kijlstra A (1990) Cytokines and intraocular inflammation. Curr Eye Res 9:207–211
de Boer JH, Van Haren MA, Baarsma GS, de Jong PV (1992) Analysis of IL-6 levels in human vitreous fluid obtained from uveitis patients, patients with proliferative intraocular disorders and eye bank eyes. Curr Eye Res 11(Suppl):181–186
Xia Q, Pang W, Pan H, Zheng Y, Kang JS, Zhu SG (2004) Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul Pept 122:173–178
Dixit VD, Taub DD (2005) Ghrelin and immunity: a young player in an old field. Exp Gerontol 40:900–910
Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C (2003) Therapeutic effects of ghrelin on endotoxic shock in rats. Eur J Pharmacol 473:171–176
Korbonits M, Ciccarelli E, Ghigo E, Grossman AB (1999) The growth hormone secretagogue receptor. Growth Hormon IGF Res 9:93–99
Pong SS, Chaung LY, Dean DC, Nargund RP, Patchett AA, Smith RG (1996) Identification of a new G-protein-linked receptor for growth hormone secretagogues. Mol Endocrinol 10:57–61
Rocha-Sousa A, Saraiva J, Henriques-Coelho T, Falcão-Reis F, Correia-Pinto J, Leite-Moreira AF (2006) Ghrelin as a novel locally produced relaxing peptide of the iris sphincter and dilator muscles. Exp Eye Res 83:1179–1187
Katsanos A, Dastiridou A, Georgoulias P, Cholevas P, Kotoula M, Tsironi EE (2011) Plasma and aqueous humour levels of ghrelin in open-angle glaucoma patients. Clin Exp Ophthalmol 39:324–329
Sehirli O, Sener E, Sener G, Cetinel S, Erzik C, Yeğen BC (2008) Ghrelin improves burn induced multiple organ injury by depressing neutrophil infiltration and the release of pro inflammatory cytokines. Peptides 29:1231–1240
Kasımay O, Işeri SO, Barlas A, Bangir D, Yeğen C, Arbak S, Yeğen BC (2006) Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res 36:11–19
Erşahin M, Toklu HZ, Erzik C, Cetinel S, Akakin D, Velioğlu-Oğünç A, Tetik S, Ozdemir ZN, Sener G, Yeğen BC (2010) The antiinflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma 27:1143–1155
Henderson DJ (1991) Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology 73:316–321
Cather JC, Abramovits W, Menter A (2001) Cyclosporine and tacrolimus in dermatology. Dermatol Clin 19:119–137
Hamalainen M, Lahti A, Moilanen E (2002) Calcineurin inhibitors, cyclosporin A and tacrolimus inhibit expression of inducible nitric oxide synthase in colon epithelial and macrophage cell lines. Eur J Pharmacol 448:239–244
Oh-i K, Keino H, Goto H, Yamakawa N, Murase K, Usui Y, Kezuka T, Sakai J, Takeuchi M, Usui M (2007) Intravitreal injection of Tacrolimus (FK506) suppresses ongoing experimental autoimmune uveoretinitis in rats. Br J Ophthalmol 91:237–242
Author Contributions
Design and conduct of study:
F. C. Gul and B. Turgut.
Collection and management of the data: B. Turgut, F. C. Gul, N. Ilhan, F. Daglı, and M. Ozgen.
Analysis and interpretation of the data: B. Turgut, F. C. Gul, N. Ilhan, F. Daglı, and M. Ozgen.
Preparation of the manuscript: B. Turgut and F.C. Gul.
Review and final approval of the manuscript: B. Turgut, F. C. Gul, N. Ilhan, F. Daglı, and M. Ozgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gül, F.C., Turgut, B., Dağlı, F. et al. The comparison of the impact of ghrelin and tacrolimus on vitreous cytokine levels in an experimental uveitis model. Graefes Arch Clin Exp Ophthalmol 251, 1235–1241 (2013). https://doi.org/10.1007/s00417-013-2259-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2259-x