Abstract
Background
Highly toxic antimetabolites have gained access to routine clinical use to modulate and reduce the amount of postoperative scarring following glaucomatous filtering procedures. It could be speculated that by combining two different antiproliferative substances with different mechanisms of action total amounts of the substances could be decreased and side effects reduced.
Methods
Twenty-two substances were tested that had antiproliferative effects by acting cytotoxically, inhibiting growth factors, or inducing apoptosis. With combinations of each two substances, cell culture experiments using 3T3 and human Tenon’s capsule fibroblasts were performed evaluating cell toxicity, proliferation and migration, the extent of free radicals, and the amount of apoptosis (TUNEL, electron microscopy). The five most potent combinations were used in an animal experiment with rabbits performing filtering procedures. The extent of episcleral scarring was evaluated by histopathology.
Results
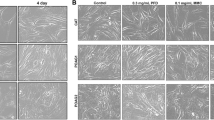
The results of the various assays revealed consistently strong effects in 5 of the 462 combinations. Of these five combinations, two were highly effective in the rabbit model. Substances with strong effects when applied in combination included staurosporine, mitomycin, and CD95L.
Conclusions
We found synergistic effects in assays that evaluated different aspects of cell function. The amount of scarring in an animal experiment was inhibited to a level comparable with a high single dose of mitomycin. Combination therapy of two antiproliferative acting substances may be a promising concept.





Similar content being viewed by others
References
Addicks EM, Quigley HA, Green WR, Robin AL (1983) Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol 101:795–798
Border WA, Noble NA (1994) Transforming growth factor β in tissue fibrosis. N Engl J Med 331:1286–1292
Buskirk van EM (1982) Cysts of Tenon’s capsule following filtration surgery. Am J Ophthalmol 94:522–527
Cairns JE (1968) Trabeculectomy: preliminary report of a new method. Am J Ophthalmol 66:673–679
Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT (2000) TGF-β1, -β2, -β3 in vitro: biphasic effects on Tenon’s fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci 41:756–763
Cordeiro MF, Reichel MB, Gay JA, D’Esposita F, Alexander RA, Khaw PT (1999) Transforming growth factor-beta1, -beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis Sci 40:1975–1982
Costa VP, Spaeth GL, Eiferman RA, Orengo-Nania S (1993) Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg 24:152–170
Costa VP, Wilson RP, Moster MR, Schmidt CM, Gandham S (1993) Hypotony maculopathy following the use of topical mitomycin C in glaucoma filtration surgery. Ophthalmic Surg 24:389–394
Cunliffe IA, Richardson PS, Rees RC, Rennie IG (1995) Effect of TNF, IL-1, and IL-6 on the proliferation of human Tenon’s capsule fibroblasts in tissue culture. Br J Ophthalmol 79:590–595
DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC (2002) Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol 120:297–300
Doxey DL, Ng MC, Dill RE, Iacopino AM (1995) Platelet-derived growth factor levels in wounds of diabetic rats. Life Sci 57:1111–1123
Drance SM, Vargas E (1973) Trabeculectomy and thermosclerostomy: a comparison of two procedures. Can J Ophthalmol 8:413–415
Fankhauser F (1992) Wound healing in glaucoma filtering surgery. Kugler, Amsterdam
Grisanti S, Gralla A, Maurer P, Diestelhorst M, Krieglstein GK, Heimann K (2000) Cellular photoablation to control postoperative fibrosis in filtration surgery: in vitro studies. Exp Eye Res 70:145–152
Grisanti S, Szurman P, Warga M, Kaczmarek R, Ziemssen F, Tatar O, Bartz-Schmidt KU (2005) Decorin modulates wound healing in experimental filtration surgery: a pilot study. Invest Ophthalmol Vis Sci 46:191–196
Hitchings RA, Grierson I (1983) Clinicopathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc U K 103:84–88
Hueber A, Esser P, Heimann K, Kociok N, Winter S, Weller M (1998) The topoisomerase I inhibitors, camptothecin and beta-lapachone, induce apoptosis of human retinal pigment epithelial cells. Exp Eye Res 67:525–530
Jampel HD, McGuigan LJB, Dunkelberger GR, L’Herault NL, Quigley HA (1988) Cellular proliferation after experimental glaucoma filtration surgery. Arch Ophthalmol 106:89–94
Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M, Sakamoto S, Miura Y (1992) Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer 20:604–610
Khaw PT, Occleston NL, Schultz G, Grierson I, Sherwood MB, Larkin G (1994) Activation and suppression of fibroblast function. Eye 8:188–195
Khaw PT, Sherwood MB, MacKay LD, Rossi MJ, Schultz G (1992) Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Arch Ophthalmol 110:1150–1154
Khaw PT, Ward S, Grierson I, Rice NSC (1991) Effect of β radiation on proliferating human Tenon’s capsule fibroblasts. Br J Ophthalmol 75:580–583
Krott R, Lebek J, Grisanti S, Esser P, Heimann K (2000) Antiproliferative effect of genistein on pig retinal pigment epithelial cells in culture. Ophthalmologica 214:296–300
Lee DA, Lee TC, Corres AE, Kitada S (1990) Effects of mithramycin, mitomycin, daunorubicin, and bleomycin on human subconjunctival fibroblast attachment and proliferation. Invest Ophthalmol Vis Sci 31:2136–2144
Lin RY, Sullivan KM, Argenta PA et al (1995) Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg 222:146–154
Maumenee AE (1960) External filtering operations for glaucoma: the mechanism of function and failure. Trans Am Ophthalmol Soc 58:319–328
Mermoud A, Salmon JF, Murray AND (1993) Trabeculectomy with mitomycin C for refractory glaucoma in blacks. Am J Ophthalmol 116:72–78
Mietz H, Chévas-Barrios P, Feldman RM, Lieberman MW (1998) Suramin inhibits wound healing following filtering procedures for glaucoma. Br J Ophthalmol 82:816–820
Mietz H, Chévaz-Barrios P, Lieberman MW, Wendt M, Gross R, Basinger SF (1997) Decorin and suramin inhibit ocular fibroblast collagen production. Graefes Arch Clin Exp Ophthalmol 235:399–403
Mietz H, Krieglstein GK (1994) Mitomycin C for trabeculectomy in complicated glaucoma: preliminary results after 6 months. Ger J Ophthalmol 3:164–167
Nouri-Mahdavi K, Brigatti L, Weitzman M, Caprioli J (1995) Outcomes of trabeculectomy for primary open-angle glaucoma. Ophthalmology 102:1760–1769
Palmer SS (1991) Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology 98:317–321
Pejnovic N, Lilic D, Zunic G et al (1995) Aberrant levels of cytokines within the healing wound after burn injury. Arch Surg 130:999–1006
Pierce GF, Tarpley JE, Tseng J et al (1995) Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest 96:1336–1350
Reber U, Wullner U, Trepel M, Baumgart J, Seyfried J, Klockgether T, Dichgans J, Weller M (1998) Potentiation of treosulfan toxicity by the glutathione-depleting agent buthionine sulfoximine in human malignant glioma cells: the role of bcl-2. Biochem Pharmacol 55:349–359
Rensing-Ehl A, Frei K, Flury R et al (1995) Local Fas/APO-1 (CD95) ligand-mediated cell killing in vivo. Eur J Immunol 25:2253–2258
Schulz JB, Weller M, Klockgether T (1996) Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci 16:4696–4706
Shields MB (1980) Trabeculectomy vs. full-thickness filtering operation for control of glaucoma. Ophthalmic Surg 11:498–505
Siriwardena D, Khaw PT, King AJ, Donaldson ML, Overton BM, Migdal C, Cordeiro MF (2002) Human antitransforming growth factor beta(2) monoclonal antibody—a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology 109:427–431
Spaeth GL, Joseph NH, Fernandez E (1975) Trabeculectomy: a re-evaluation after three years and a comparison with Scheie’s procedure. Trans Am Acad Ophthalmol Otolaryngol 79:349–361
Sullivan KM, Lorenz HP, Meuli M, Lin RY, Adzick NS (1995) A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg 30:198–203
Tahery MM, Lee DA (1989) Pharmacologic control of wound healing in glaucoma filtration surgery. J Ocul Pharmacol 5:155–179
Teng CC, Chi HH, Katzin HM (1959) Histology and mechanism of filtering operations. Am J Ophthalmol 47:16–34
The fluorouracil filtering study group (1996) Five-year follow-up of the Fluorouracil Filtering Surgery Study. The Fluorouracil Filtering Surgery Study Group. Am J Ophthalmol 121:349–366
Thomas DW, O’Neill ID, Harding KG, Shepherd JP (1995) Cutaneous wound healing: a current perspective. J Oral Maxillofac Surg 53:442–447
Watson P (1970) Trabeculectomy. A modified ab externo technique. Ann Ophthalmol 2:199–205
Webb JL (1963) Effect of more than one inhibitor. In: Webb JL (ed) Enzyme and metabolic inhibitors, Vol. 1. Academic, New York, pp 66–79, 487–512
Weller M, Frei K, Groscurth P, Krammer PH, Yonekawa Y, Fontana A (1994) J Clin Invest 94:954–964
Yamamoto T, Varani J, Soong HK, Lichter PR (1990) Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology 97:1204–1210
Acknowledgement
This study was supported in part by DFG, Bonn, Germany Mi 347/5-1, and Koeln fortune program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mietz, H., Welsandt, G., Hueber, A. et al. Synergistic effects of combined cytotoxic and apoptosis-inducing drugs on Tenon’s capsule fibroblasts in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol 245, 1367–1375 (2007). https://doi.org/10.1007/s00417-007-0547-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0547-z