Abstract
Background
Virotherapy represents a novel therapeutic modality for the treatment of malignant diseases. Vesicular stomatitis virus (VSV) has been shown to exert antitumor effect in several tumor types. Since the potential oncolytic activity of VSV has not yet been evaluated in epithelial tumors of the conjunctiva, we set out to investigate the susceptibility of the immortalized Wong−Kilbourne derivative of the Chang conjunctival cell line (WK) to VSV and analyze the role of apoptosis in VSV-mediated induction of cell death.
Methods
WK cells were infected with VSV at various multiplicities and maintained for different periods of time. VSV-infected cells were analyzed by inverted microscopy for the development of cytopathic effects (CPE). Virus replication was measured by indirect immunofluorescence assay, Western blot analysis and plaque titration. The apoptotic response of the infected cells was quantitated by ELISA detecting the enrichment of nucleosomes in the cytoplasm. Western blot analysis was used to determine the levels of Bcl-2 and Bax proteins.
Results
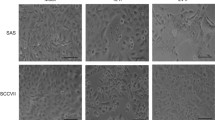
The WK cell line was highly permissive to VSV replication and was highly susceptible for the CPE of this virus. VSV infection elicited the apoptotic death of WK cells. Mock-infected cells exhibited endogenous expression of Bcl-2 and p21 Bax proteins. VSV infection caused a significant decrease in the expression level of Bcl-2. Moreover, in parallel with a slight decrease in the level of p21 Bax, p18 Bax protein accumulated in VSV-infected WK cells.
Conclusions
VSV is a powerful inducer of apoptosis in immortalized WK cells. The VSV-mediated alterations in the expressions of Bcl-2 and Bax proteins may play important roles in the apoptotic responses of infected cells and may also sensitize to other apoptotic stimuli. This virus may possess oncolytic activity in epithelial tumors of the conjunctiva.





Similar content being viewed by others
References
Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN (2000) Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol 74:1513–1523
Balachandran S, Porosnicu M, Barber GN (2001) Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves the induction of apoptosis. J Virol 75:3474–3479
Balachandran S, Barber GN (2004) Defective translation control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51–65
Basti S, Macsai MS (2003) Ocular surface squamous neoplasia: a review. Cornea 22:687–704
Cao X, Deng X, May S (2003) Cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and cathepsin-like protease may rapidly degrade p18 Bax. Blood 102:2605–2614
Cartron P–F, Oliver L, Juin P, Meflah K, Vallette FM (2004) The p18 truncated form of Bax behaves like a Bcl-2 homology domain 3-only protein. J Biol Chem 279:11503–11512
Debatin KM (2004) Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother 53:153–159
De Saint Jean M, Baudouin C, Di Nolfo M, Roman S, Lozato P, Warnet JM, Brignole F (2004) Comparison of morphological and functional characteristics of primary-cultured human conjunctival epithelium and of Wong–Kilbourne derivative of Chang conjunctival cell line. Exp Eye Res 78:257–274
Ebert O, Shinozaki K, Huang TG (2003) Oncolytic vesicular stomatitis virus for treatment of orthopic hepatocellular carcinoma in immune-competent rats. Cancer Res 63:3605–3611
Ebert O, Shinozaki K, Kournioti C, Park MS, García-Sastre A, Woo SL (2004) Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res 64:3265–3270
Fernandez M, Porosnicu M, Markovic D, Barber GN (2002) Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol 76:895–904
Huang TG, Ebert O, Shinozaki K, Garcia-Sastre A, Woo SL (2003) Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immunocompetent mice. Mol Ther 8:434–440
Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M (2000) Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA 97:10872–10877
Kirn DH (2000) Replication-selective microbiological agents: fighting cancer with targeted germ warfare. J Clin Invest 105:837–839
Kopecky SA, Willingham MC, Lyles DS (2001) Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol 75:12169–12181
Kopecky SA, Lyles DS (2003) Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol 77:4658–4669
Kopecky SA, Lyles DS (2003) The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death. J Virol 77:5524–5528
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183–192
Letchworth GJ, Rodriguez LL, Del C, Barrera J (1999) Vesicular stomatitis. Vet J 157:239–260
Lichty BD, Power AT, Stojdl DF, Bell JC (2004) Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med 10:210–216
Lichty BD, Stojdl DF, Taylor RA, Miller L, Frenkel I, Atkins H, Bell JC (2004) Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Hum Gene Ther 15:821–831
Lyles DS (2000) Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol Mol Biol Rev 64:709–724
Obuchi M, Fernandez M, Barber GN (2003) Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J.Virol 77:8843–8856
Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed JC, Miyawaki T (1995) Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol 7:1817–1825
Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE (2000) The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complex. Mol Cell Biol 20:8590–8601
Re GG, Hazen–Martin DJ, El Bahtimi R, Brownlee NA, Willingham MC, Garvin AJ (1999) Prognostic significance of Bcl-2 in Wilms′ tumor and oncogenic potential of Bcl-X(L) in rare tumor cases. Int J Cancer 84:192–200
Ring CJA (2002) Cytolytic viruses as potential anti-cancer agents. J Gen Virol 83:491–502
Rodriguez LL, Pauszek SJ, Bunch TA, Schumann KR (2002) Full-length genome analysis of natural isolates of vesicular stomatitis virus (Indiana 1 serotype) from North, Central and South America. J Gen Virol 83:2475–2483
Rose JK, Whitt MA (2001) Rhabdoviridae: the viruses and their replication. In: Knipe DM, Howley PM (eds) Fields virology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1221–1242
Shields CL, Shields JA (2004) Tumors of the conjunctiva and cornea. Surv Ophthalmol 49:3–24
Shinozaki K, Ebert O, Kournioti C, Tai YS, Woo SL (2004) Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol Ther 9:368–376
Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC (2000) Exploiting tumor-specific defects in the interferon pathway with previously unknown oncolytic virus. Nat Med 6:821–825
Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC (2000) The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol 74:9580–9585
Stojdl DF, Lichty BD, tenOver BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC (2003) VSV strains with defects in their ability to shutdown innate immunity are potent systemic anticancer agents. Cancer Cell 4:263–275
Verma IM, Weitzman MD (2005) Gene therapy: twenty-first century medicine. Annu Rev Biochem 74:711–738
Wilson MW, Czechonska G, Finger PT, Rausen A, Hooper ME, Haik BG (2001) Chemotherapy for eye cancer. Surv Ophthalmol 45:416–444
Acknowledgements
We thank Gyöngyi Ábrahám for expert technical assistance. This study was supported by grants ETT/398/2003 from the Hungarian Ministry of Health, Social and Family Affairs and OTKA/T043144 from the Hungarian Scientific Research Fund. Klára Megyeri is a recipient of a grant from the Bolyai Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallyas, É., Seprényi, G., Sonkoly, E. et al. Vesicular stomatitis virus induces apoptosis in the Wong–Kilbourne derivative of the Chang conjunctival cell line. Graefe's Arch Clin Exp Ophthalmo 244, 717–724 (2006). https://doi.org/10.1007/s00417-005-0162-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0162-9