Abstract
Background
Nearly 1 million Americans are living with multiple sclerosis (MS) and 30–50% will experience memory dysfunction. It remains unclear whether this memory dysfunction is due to overall white matter lesion burden or damage to specific neuroanatomical structures. Here we test if MS memory dysfunction is associated with white matter lesions to a specific brain circuit.
Methods
We performed a cross-sectional analysis of standard structural images and verbal memory scores as assessed by immediate recall trials from 431 patients with MS (mean age 49.2 years, 71.9% female) enrolled at a large, academic referral center. White matter lesion locations from each patient were mapped using a validated algorithm. First, we tested for associations between memory dysfunction and total MS lesion volume. Second, we tested for associations between memory dysfunction and lesion intersection with an a priori memory circuit derived from stroke lesions. Third, we performed mediation analyses to determine which variable was most associated with memory dysfunction. Finally, we performed a data-driven analysis to derive de-novo brain circuits for MS memory dysfunction using both functional (n = 1000) and structural (n = 178) connectomes.
Results
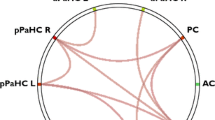
Both total lesion volume (r = 0.31, p < 0.001) and lesion damage to our a priori memory circuit (r = 0.34, p < 0.001) were associated with memory dysfunction. However, lesion damage to the memory circuit fully mediated the association of lesion volume with memory performance. Our data-driven analysis identified multiple connections associated with memory dysfunction, including peaks in the hippocampus (T = 6.05, family-wise error p = 0.000008), parahippocampus, fornix and cingulate. Finally, the overall topography of our data-driven MS memory circuit matched our a priori stroke-derived memory circuit.
Conclusions
Lesion locations associated with memory dysfunction in MS map onto a specific brain circuit centered on the hippocampus. Lesion damage to this circuit fully mediated associations between lesion volume and memory. A circuit-based approach to mapping MS symptoms based on lesions visible on standard structural imaging may prove useful for localization and prognosis of higher order deficits in MS.




Similar content being viewed by others
References
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19:860–871
Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, Weinstock-Guttman B (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 12:549–558
De Meo E, Portaccio E, Giorgio A, Ruano L, Goretti B, Niccolai C, Patti F, Chisari CG, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Stampatori C, Simone M, Viterbo RG, Bonacchi R, Rocca MA, De Stefano N, Filippi M, Amato MP (2021) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78:414–425
McGinley MP, Goldschmidt CH, Rae-Grant AD (2021) Diagnosis and treatment of multiple sclerosis: a review. JAMA 325:765–779
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Sormani MP, Bruzzi P (2013) MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 12:669–676
Swirsky-Sacchetti T, Mitchell DR, Seward J, Gonzales C, Lublin F, Knobler R, Field HL (1992) Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology 42:1291–1295
Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L (1989) Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 39:161–166
Uher T, Krasensky J, Sobisek L, Blahova Dusankova J, Seidl Z, Kubala Havrdova E, Sormani MP, Horakova D, Kalincik T, Vaneckova M (2018) Cognitive clinico-radiological paradox in early stages of multiple sclerosis. Ann Clin Transl Neurol 5:81–91
Mollison D, Sellar R, Bastin M, Mollison D, Chandran S, Wardlaw J, Connick P (2017) The clinico-radiological paradox of cognitive function and MRI burden of white matter lesions in people with multiple sclerosis: a systematic review and meta-analysis. PLoS One 12:e0177727
Rossi F, Giorgio A, Battaglini M, Stromillo ML, Portaccio E, Goretti B, Federico A, Hakiki B, Amato MP, De Stefano N (2012) Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS One 7:e44826
Izquierdo G, Campoy F Jr, Mir J, Gonzalez M, Martinez-Parra C (1991) Memory and learning disturbances in multiple sclerosis. MRI lesions and neuropsychological correlation. Eur J Radiol 13:220–224
Roosendaal SD, Moraal B, Pouwels PJ, Vrenken H, Castelijns JA, Barkhof F, Geurts JJ (2009) Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 15:708–714
Sacco R, Bisecco A, Corbo D, Della Corte M, d’Ambrosio A, Docimo R, Gallo A, Esposito F, Esposito S, Cirillo M, Lavorgna L, Tedeschi G, Bonavita S (2015) Cognitive impairment and memory disorders in relapsing-remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol 262:1691–1697
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, Rovira A, Sumowski JF, Valsasina P, Filippi M, Group MS (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14:302–317
Tedeschi G, Dinacci D, Lavorgna L, Prinster A, Savettieri G, Quattrone A, Livrea P, Messina C, Reggio A, Servillo G, Bresciamorra V, Orefice G, Paciello M, Brunetti A, Paolillo A, Coniglio G, Bonavita S, Di Costanzo A, Bellacosa A, Valentino P, Quarantelli M, Patti F, Salemi G, Cammarata E, Simone I, Salvatore M, Bonavita V, Alfano B (2007) Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci 263:15–19
Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C (2014) The link between multiple sclerosis and depression. Nat Rev Neurol 10:507–517
Brandstadter R, Ayeni O, Krieger SC, Harel NY, Escalon MX, Katz Sand I, Leavitt VM, Fabian MT, Buyukturkoglu K, Klineova S, Riley CS, Lublin FD, Miller AE, Sumowski JF (2020) Detection of subtle gait disturbance and future fall risk in early multiple sclerosis. Neurology 94:e1395–e1406
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15:239–245
Engl C, Tiemann L, Grahl S, Bussas M, Schmidt P, Pongratz V, Berthele A, Beer A, Gaser C, Kirschke JS, Zimmer C, Hemmer B, Muhlau M (2020) Cognitive impairment in early MS: contribution of white matter lesions, deep grey matter atrophy, and cortical atrophy. J Neurol 267:2307–2318
Rovaris M, Filippi M, Falautano M, Minicucci L, Rocca MA, Martinelli V, Comi G (1998) Relation between MR abnormalities and patterns of cognitive impairment in multiple sclerosis. Neurology 50:1601–1608
Deloire MS, Salort E, Bonnet M, Arimone Y, Boudineau M, Amieva H, Barroso B, Ouallet JC, Pachai C, Galliaud E, Petry KG, Dousset V, Fabrigoule C, Brochet B (2005) Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76:519–526
Mesaros S, Rocca MA, Riccitelli G, Pagani E, Rovaris M, Caputo D, Ghezzi A, Capra R, Bertolotto A, Comi G, Filippi M (2009) Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp 30:2656–2666
Sperling RA, Guttmann CR, Hohol MJ, Warfield SK, Jakab M, Parente M, Diamond EL, Daffner KR, Olek MJ, Orav EJ, Kikinis R, Jolesz FA, Weiner HL (2001) Regional magnetic resonance imaging lesion burden and cognitive function in multiple sclerosis: a longitudinal study. Arch Neurol 58:115–121
Brainin M, Goldenberg G, Ahlers C, Reisner T, Neuhold A, Deecke L (1988) Structural brain correlates of anterograde memory deficits in multiple sclerosis. J Neurol 235:362–365
Altermatt A, Gaetano L, Magon S, Haring DA, Tomic D, Wuerfel J, Radue EW, Kappos L, Sprenger T (2018) Clinical correlations of brain lesion location in multiple sclerosis: voxel-based analysis of a large clinical trial dataset. Brain Topogr 31:886–894
Weaver NA, Kuijf HJ, Aben HP, Abrigo J, Bae HJ, Barbay M, Best JG, Bordet R, Chappell FM, Chen C, Dondaine T, van der Giessen RS, Godefroy O, Gyanwali B, Hamilton OKL, Hilal S, Huenges Wajer IMC, Kang Y, Kappelle LJ, Kim BJ, Kohler S, de Kort PLM, Koudstaal PJ, Kuchcinski G, Lam BYK, Lee BC, Lee KJ, Lim JS, Lopes R, Makin SDJ, Mendyk AM, Mok VCT, Oh MS, van Oostenbrugge RJ, Roussel M, Shi L, Staals J, Del CV-HM, Venketasubramanian N, Verhey FRJ, Wardlaw JM, Werring DJ, Xin X, Yu KH, van Zandvoort MJE, Zhao L, Biesbroek JM, Biessels GJ (2021) Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 20:448–459
Biesbroek JM, van Zandvoort MJ, Kappelle LJ, Schoo L, Kuijf HJ, Velthuis BK, Biessels GJ, Postma A, Utrecht VCI (2015) Distinct anatomical correlates of discriminability and criterion setting in verbal recognition memory revealed by lesion-symptom mapping. Hum Brain Mapp 36:1292–1303
Fox MD (2018) Mapping symptoms to brain networks with the human connectome. N Engl J Med 379:2237–2245
Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr, Fox MD (2015) Network localization of neurological symptoms from focal brain lesions. Brain 138:3061–3075
Joutsa J, Corp DT, Fox MD (2022) Lesion network mapping for symptom localization: recent developments and future directions. Curr Opin Neurol 35:453–459
Kletenik I, Ferguson MA, Bateman JR, Cohen AL, Lin C, Tetreault A, Pelak VS, Anderson CA, Prasad S, Darby RR, Fox MD (2022) Network localization of unconscious visual perception in blindsight. Ann Neurol 91:217–224
Kletenik I, Gaudet K, Prasad S, Cohen AL, Fox MD (2023) Network localization of awareness in visual and motor anosognosia. Ann Neurol. https://doi.org/10.1002/ana.26709
Cohen AL, Mulder BPF, Prohl AK, Soussand L, Davis P, Kroeck MR, McManus P, Gholipour A, Scherrer B, Bebin EM, Wu JY, Northrup H, Krueger DA, Sahin M, Warfield SK, Fox MD, Peters JM, Tuberous Sclerosis Complex Autism Center of Excellence Network Study G (2021) Tuber locations associated with infantile spasms map to a common brain network. Ann Neurol 89:726–739
Ferguson MA, Lim C, Cooke D, Darby RR, Wu O, Rost NS, Corbetta M, Grafman J, Fox MD (2019) A human memory circuit derived from brain lesions causing amnesia. Nat Commun 10:3497
Siddiqi SH, Schaper F, Horn A, Hsu J, Padmanabhan JL, Brodtmann A, Cash RFH, Corbetta M, Choi KS, Dougherty DD, Egorova N, Fitzgerald PB, George MS, Gozzi SA, Irmen F, Kuhn AA, Johnson KA, Naidech AM, Pascual-Leone A, Phan TG, Rouhl RPW, Taylor SF, Voss JL, Zalesky A, Grafman JH, Mayberg HS, Fox MD (2021) Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav 5:1707–1716
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, Ross TJ, Deshpande HU, Wang HZ, Bruss J, Stein EA, Volkow ND, Grafman JH, van Wijngaarden E, Boes AD, Fox MD (2022) Brain lesions disrupting addiction map to a common human brain circuit. Nat Med 28:1249–1255
Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50
Wattjes MP, Rovira A, Miller D, Yousry TA, Sormani MP, de Stefano MP, Tintore M, Auger C, Tur C, Filippi M, Rocca MA, Fazekas F, Kappos L, Polman C, Frederik B, Xavier M, Group MS (2015) Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol 11:597–606
Tedone N, Preziosa P, Meani A, Pagani E, Vizzino C, Filippi M, Rocca MA (2023) Regional white matter and gray matter damage and cognitive performances in multiple sclerosis according to sex. Mol Psychiatry 28:1783–1792
Grajauskas LA, Frizzell T, Song X, D’Arcy RCN (2019) White matter fMRI activation cannot be treated as a nuisance regressor: overcoming a historical blind spot. Front Neurosci 13:1024
Siddiqi SH, Kletenik I, Anderson MC, Cavallari M, Chitnis T, Glanz BI, Khalil S, Palotai M, Bakshi R, Guttmann CRG, Fox MD (2023) Lesion network localization of depression in multiple sclerosis. Nat Ment Health 1:36–44
Healy BC, Zurawski J, Gonzalez CT, Chitnis T, Weiner HL, Glanz BI (2019) Assessment of computer adaptive testing version of the Neuro-QOL for people with multiple sclerosis. Mult Scler 25:1791–1799
Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, Hämäläinen P, Hartung HP, Krupp L, Penner IK, Reder AT, Benedict RH (2012) Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler 18:891–898
Stegen S, Stepanov I, Cookfair D, Schwartz E, Hojnacki D, Weinstock-Guttman B, Benedict RH (2010) Validity of the California verbal learning test-II in multiple sclerosis. Clin Neuropsychol 24:189–202
Fink F, Eling P, Rischkau E, Beyer N, Tomandl B, Klein J, Hildebrandt H (2010) The association between California Verbal Learning Test performance and fibre impairment in multiple sclerosis: evidence from diffusion tensor imaging. Mult Scler 16:332–341
Lafosse JM, Mitchell SM, Corboy JR, Filley CM (2013) The nature of verbal memory impairment in multiple sclerosis: a list-learning and meta-analytic study. J Int Neuropsychol Soc 19:995–1008
Meier DS, Guttmann CRG, Tummala S, Moscufo N, Cavallari M, Tauhid S, Bakshi R, Weiner HL (2018) Dual-sensitivity multiple sclerosis lesion and CSF segmentation for multichannel 3T brain MRI. J Neuroimaging 28:36–47
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205
Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson M, Darby RR, Soussand L, Horn A, Kim NY, Voss JL, Naidech AM, Brodtmann A, Egorova N, Gozzi S, Phan TG, Corbetta M, Grafman J, Fox MD (2019) A human depression circuit derived from focal brain lesions. Biol Psychiatry 86:749–758
Zhao SZ, Zhao YX, Liao XH, Huo R, Li H, Jiao YM, Weng JC, Wang J, Liu B, Cao Y (2023) Unruptured brain arteriovenous malformations causing seizures localize to one common brain network. J Neurosci Res 101:245–255
Sharma J, Sanfilipo MP, Benedict RH, Weinstock-Guttman B, Munschauer FE 3rd, Bakshi R (2004) Whole-brain atrophy in multiple sclerosis measured by automated versus semiautomated MR imaging segmentation. AJNR Am J Neuroradiol 25:985–996
Sotiropoulos MG, Lokhande H, Healy BC, Polgar-Turcsanyi M, Glanz BI, Bakshi R, Weiner HL, Chitnis T (2021) Relapse recovery in multiple sclerosis: effect of treatment and contribution to long-term disability. Mult Scler J Exp Transl Clin 7:20552173211015504
Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, Multiple Sclerosis Outcome Assessments C (2017) Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 23:721–733
Lopez-Gongora M, Querol L, Escartin A (2015) A one-year follow-up study of the Symbol Digit Modalities Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) in relapsing-remitting multiple sclerosis: an appraisal of comparative longitudinal sensitivity. BMC Neurol 15:40
Hayes AF (2022) Introduction to mediation, moderation, and conditional process analysis, third edition: a regression-based approach. Guilford Publications, New York
Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG (2006) Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 67:2176–2185
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397
Foulon C, Cerliani L, Kinkingnehun S, Levy R, Rosso C, Urbanski M, Volle E, Thiebaut de Schotten M (2018) Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience 7:1–17
Vu AT, Auerbach E, Lenglet C, Moeller S, Sotiropoulos SN, Jbabdi S, Andersson J, Yacoub E, Ugurbil K (2015) High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. Neuroimage 122:318–331
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790
Chard D, Trip SA (2017) Resolving the clinico-radiological paradox in multiple sclerosis. F1000Res 6:1828
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Sestieri C, Corbetta M, Romani GL, Shulman GL (2011) Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci 31:4407–4420
Valdes Cabrera D, Smyth P, Blevins G, Emery D, Beaulieu C (2022) Diffusion imaging of fornix and interconnected limbic deep grey matter is linked to cognitive impairment in multiple sclerosis. Eur J Neurosci 55:277–294
Poppenk J, McIntosh AR, Craik FI, Moscovitch M (2010) Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci 30:4707–4716
Poppenk J, Kohler S, Moscovitch M (2010) Revisiting the novelty effect: when familiarity, not novelty, enhances memory. J Exp Psychol Learn Mem Cogn 36:1321–1330
Bowren M, Bruss J, Manzel K, Edwards D, Liu C, Corbetta M, Tranel D, Boes AD (2022) Post-stroke outcomes predicted from multivariate lesion-behaviour and lesion network mapping. Brain 145:1338–1353
Crockett RA, Hsu CL, Dao E, Tam R, Eng JJ, Handy TC, Liu-Ambrose T (2021) Painting by lesions: white matter hyperintensities disrupt functional networks and global cognition. Neuroimage 236:118089
Lie IA, Weeda MM, Mattiesing RM, Mol MAE, Pouwels PJW, Barkhof F, Torkildsen O, Bo L, Myhr KM, Vrenken H (2022) Relationship between white matter lesions and gray matter atrophy in multiple sclerosis: a systematic review. Neurology 98:e1562–e1573
Albert MS, Moss MB, Tanzi R, Jones K (2001) Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 7:631–639
Gavett BE, Horwitz JE (2012) Immediate list recall as a measure of short-term episodic memory: insights from the serial position effect and item response theory. Arch Clin Neuropsychol 27:125–135
Casaletto KB, Marx G, Dutt S, Neuhaus J, Saloner R, Kritikos L, Miller B, Kramer JH (2017) Is “Learning” episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia 102:19–28
Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M (2010) Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 74:1252–1259
Tona F, Petsas N, Sbardella E, Prosperini L, Carmellini M, Pozzilli C, Pantano P (2014) Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology 271:814–821
Acknowledgements
The authors thank the patients who participated in the SysteMS study and the research staff at the Brigham MS Center for assistance, especially Mark Anderson. The authors thank Dr. Margaret O’Connor and Dr. Rebecca Amariglio for their advice and guidance. The authors thank the members of the Center for Brain Circuit Therapeutics for assistance especially Christopher Lin. Data were provided, in part, by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Funding
AC was funded by NIH/NIMH K23MH120510, the Child Neurology Foundation, and the Simons Foundation Autism Research Initiative. M.D.F. was supported by the Nancy Lurie Marks Foundation, the Mather’s Foundation, the Ellison/Baszucki Foundation, the Kaye Family Research Endowment and National Institutes of Health grants R21 MH126271, R56 AG069086, R01 MH113929, R01 MH115949, and R01 AG060987. The SysteMS cohort of the CLIMB study was supported in part by Verily Life Sciences. The CLIMB study is supported in part by the Watercove Foundation.
Author information
Authors and Affiliations
Contributions
Study concept and design: all authors; data acquisition and analysis: all authors; drafting the text or figures: IK, ALC, RB, MDF.
Corresponding author
Ethics declarations
Conflicts of interest
Rohit Bakshi has received consulting fees from Bristol-Myers Squibb and EMD Serono and research support from Bristol-Myers Squibb, EMD Serono, and Novartis. The other authors report no competing interests.
Ethical standards
The study was approved by Mass General Brigham/Partners Institutional Review Board Protocols 2015P001248, 2020P002987 and 2020P000737 and all participants provided written informed consent. This study has been approved by the appropriate ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Data availability statement
The functional connectivity data equivalent to that used in this study is available online through the Harvard Dataverse at: https://doi.org/10.7910/DVN/ILXIKS and the pipeline used to prepare the functional connectivity data is available at: https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016. The code to prepare structural connectivity maps is available at: http://www.bcblab.com/BCB/Disconnectome.html and the structural connectivity data is available at: https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release. Statistical analyses were performed in MatLab (version 2019b) or SPSS (version 27.0.1.0). MS lesion data is available for review upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kletenik, I., Cohen, A.L., Glanz, B.I. et al. Multiple sclerosis lesions that impair memory map to a connected memory circuit. J Neurol 270, 5211–5222 (2023). https://doi.org/10.1007/s00415-023-11907-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11907-8