Abstract
Background
Parkinson's disease (PD) is characterized by a lateralized onset, but its cause and mechanism are still unclear.
Methods
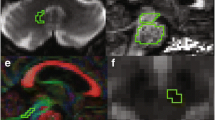
Obtaining diffusion tensor imaging (DTI) data from the Parkinson’s Progression Markers Initiative (PPMI). Tract-based spatial statistics analysis and region-of-interest-based analysis were performed to evaluate the white matter (WM) asymmetry using original DTI parameters, Z Score normalized parameters, or the asymmetry index (AI). Hierarchical cluster analysis and least absolute shrinkage and selection operator regression were performed to construct predictive models for predicting the PD onset side. DTI data from The Second Affiliated Hospital of Chongqing Medical University were obtained for external validation of the prediction model.
Results
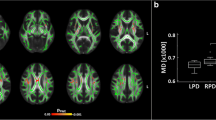
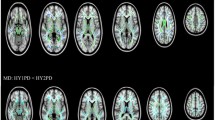
118 PD patients and 69 healthy controls (HC) from PPMI were included. Right-onset PD patients presented more asymmetric areas than left-onset PD patients. The inferior cerebellar peduncle (ICP), superior cerebellar peduncle (SCP), external capsule (EC), cingulate gyrus (CG), superior fronto-occipital fasciculus (SFO), uncinate fasciculus (UNC), and tapetum (TAP) showed significant asymmetry in left-onset and right-onset PD patients. An onset-side-specific pattern of WM alterations exists in PD patients, and a prediction model was constructed. The predicting models based on AI and ΔZ Score presented favorable efficacy in predicting PD onset side by external validation in 26 PD patients and 16 HCs from our hospital.
Conclusions
Right-onset PD patients may have more severe WM damage than left-onset PD patients. WM asymmetry in ICP, SCP, EC, CG, SFO, UNC, and TAP may predict PD onset side. Imbalances in the WM network may underlie the mechanism of lateralized onset in PD.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors.
References
Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: Present and future. Metabolism 64:S40–S46. https://doi.org/10.1016/j.metabol.2014.10.030
Wang J, Yang QX, Sun X et al (2015) MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson’s disease. Parkinsonism Relat Disord 21:590–596. https://doi.org/10.1016/j.parkreldis.2015.03.012
Djaldetti R, Ziv I, Melamed E (2006) The mystery of motor asymmetry in Parkinson’s disease. The Lancet Neurology 5:796–802. https://doi.org/10.1016/S1474-4422(06)70549-X
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Özekmekçi S, Benbir G, Özdogan FY et al (2007) Hemihypomimia, a rare persistent sign in Parkinson’s disease: follow up of 11 patients. J Neurol 254:347–350. https://doi.org/10.1007/s00415-006-0372-z
Guerra-Hiraldo JD, López-Jiménez A, Gasca-Salas C et al (2023) Hemihypomimia in Parkinson’s disease: an under-recognized clinical sign? J Neurol 270:548–551. https://doi.org/10.1007/s00415-022-11292-8
Djaldetti R, Shifrin A, Rogowski Z et al (2004) Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 62:2171–2175. https://doi.org/10.1212/01.WNL.0000130455.38550.9D
Fusina S, Conte S, Bertolasi L et al (1999) Sympathetic skin response asymmetry in early stage idiopathic Parkinson’s disease. Clin Neurophysiol 110:358–366. https://doi.org/10.1016/S1388-2457(98)00012-1
Quinn NP, Lang AE, Koller WC, Marsden CD (1986) PAINFUL PARKINSON’S DISEASE. The Lancet 327:1366–1369. https://doi.org/10.1016/S0140-6736(86)91674-0
Snider SR, Fahn S, Isgreen WP, Cote LJ (1976) Primary sensory symptoms in parkinsonism. Neurology 26:423–429. https://doi.org/10.1212/wnl.26.5.423
Levin J, Kurz A, Arzberger T et al (2016) The Differential Diagnosis and Treatment of Atypical Parkinsonism. Dtsch Arztebl Int. https://doi.org/10.3238/arztebl.2016.0061
Ham JH, Lee JJ, Kim JS et al (2015) Is dominant-side onset associated with a better motor compensation in Parkinson’s disease? Mov Disord 30:1921–1925. https://doi.org/10.1002/mds.26418
Sawle GV, Playford ED, Brooks DJ et al (1993) Asymmetrical pre-synaptic and post-synaptic changes in the striatal dopamine projection in dopa naïve parkinsonism: diagnostic implications of the D2 receptor status. Brain 116:853–867. https://doi.org/10.1093/brain/116.4.853
Pelizzari L, Di Tella S, Laganà MM et al (2020) White matter alterations in early Parkinson’s disease: role of motor symptom lateralization. Neurol Sci 41:357–364. https://doi.org/10.1007/s10072-019-04084-y
Marek K, Jennings D, Lasch S et al (2011) The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 95:629–635. https://doi.org/10.1016/j.pneurobio.2011.09.005
Jenkinson M, Beckmann CF, Behrens TEJ et al (2012) FSL NeuroImage 62:782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Winkler AM, Ridgway GR, Webster MA et al (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060
Mori S, Oishi K, Jiang H et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582. https://doi.org/10.1016/j.neuroimage.2007.12.035
Boscolo Galazzo I, Mattoli MV, Pizzini FB, et al (2016) Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of 18 F-FDG PET and arterial spin labeling. NeuroImage: Clinical 11:648–657. https://doi.org/10.1016/j.nicl.2016.04.005
Carper RA, Treiber JM, DeJesus SY, Müller R-A (2016) Reduced hemispheric asymmetry of white matter microstructure in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 55:1073–1080. https://doi.org/10.1016/j.jaac.2016.09.491
Riederer P, Sian-Hülsmann J (2012) The significance of neuronal lateralisation in Parkinson’s disease. J Neural Transm 119:953–962. https://doi.org/10.1007/s00702-012-0775-1
Baumann CR, Held U, Valko PO et al (2014) Body side and predominant motor features at the onset of Parkinson’s disease are linked to motor and nonmotor progression: Predictors of PD Motor and Nonmotor Outcomes. Mov Disord 29:207–213. https://doi.org/10.1002/mds.25650
Munhoz RP, Espay AJ, Morgante F et al (2013) Long-duration Parkinson’s disease: Role of lateralization of motor features. Parkinsonism Relat Disord 19:77–80. https://doi.org/10.1016/j.parkreldis.2012.07.008
Frazzitta G, Ferrazzoli D, Maestri R, et al (2015) Differences in Muscle Strength in Parkinsonian Patients Affected on the Right and Left Side. PLoS ONE 10:e0121251. https://doi.org/10.1371/journal.pone.0121251
Rektor I, Svátková A, Vojtíšek L, et al (2018) White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS ONE 13:e0187939. https://doi.org/10.1371/journal.pone.0187939
Scherfler C, Seppi K, Mair KJ et al (2012) Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain 135:3348–3354. https://doi.org/10.1093/brain/aws253
Iranzo A, Stefani A, Niñerola-Baizan A et al (2020) Left-hemispheric predominance of nigrostriatal deficit in isolated REM sleep behavior disorder. Neurology 94:e1605–e1613. https://doi.org/10.1212/WNL.0000000000009246
Kim JS, Yang J, Lee J et al (2014) Topographic pattern of cortical thinning with consideration of motor laterality in Parkinson disease. Parkinsonism Relat Disord 20:1186–1190. https://doi.org/10.1016/j.parkreldis.2014.08.021
Thiebaut de Schotten M, Forkel SJ (2022) The emergent properties of the connected brain. Science 378:505–510. https://doi.org/10.1126/science.abq2591
Standring S (2008) Gray’s Anatomy, 40th edn. Churchill Livingstone, London
Zhong Y, Liu H, Liu G, et al (2022) A review on pathology, mechanism, and therapy for cerebellum and tremor in Parkinson’s disease. npj Parkinsons Dis 8:82. https://doi.org/10.1038/s41531-022-00347-2
Ogawa T, Hatano T, Kamagata K et al (2021) White matter alterations in Parkinson’s disease with levodopa-induced dyskinesia. Parkinsonism Relat Disord 90:8–14. https://doi.org/10.1016/j.parkreldis.2021.07.021
Shen Q, Liu Y, Guo J et al (2022) Impaired white matter microstructure associated with severe depressive symptoms in patients with PD. Brain Imaging Behav 16:169–175. https://doi.org/10.1007/s11682-021-00488-7
Uhr L, Tsolaki E, Pouratian N (2022) Diffusion tensor imaging correlates of depressive symptoms in Parkinson disease. J of Comparative Neurology 530:1729–1738. https://doi.org/10.1002/cne.25310
Takeshige-Amano H, Hatano T, Kamagata K et al (2022) White matter microstructures in Parkinson’s disease with and without impulse control behaviors. Ann Clin Transl Neurol 9:253–263. https://doi.org/10.1002/acn3.51504
Wang J, Zhang F, Zhao C, et al (2020) Investigation of local white matter abnormality in Parkinson’s disease by using an automatic fiber tract parcellation. Behavioural Brain Research 394:112805. https://doi.org/10.1016/j.bbr.2020.112805
Lucas-Jiménez O, Díez-Cirarda M, Ojedaa N et al (2015) Verbal memory in parkinson’s disease: a combined DTI and fMRI study. J Parkinsons Dis 5:793–804. https://doi.org/10.3233/jpd-150623
Di Tella S, Baglio F, Pelizzari L, et al (2020) Uncinate fasciculus and word selection processing in Parkinson’s disease. Neuropsychologia 146:107504. https://doi.org/10.1016/j.neuropsychologia.2020.107504
Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev 92:104–127. https://doi.org/10.1016/j.neubiorev.2018.05.008
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–589. https://doi.org/10.1038/nrn1432
Kantarci K, Senjem ML, Avula R et al (2011) Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 77:26–34. https://doi.org/10.1212/WNL.0b013e31822313dc
Aarsland D, Batzu L, Halliday GM et al (2021) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7:47. https://doi.org/10.1038/s41572-021-00280-3
Lally H, Hart AR, Bay AA et al (2020) Association between motor subtype and visuospatial and executive function in mild-moderate Parkinson disease. Arch Phys Med Rehabil 101:1580–1589. https://doi.org/10.1016/j.apmr.2020.05.018
Du J, Zhu H, Yu L, et al (2021) Multi-Dimensional Diffusion Tensor Imaging Biomarkers for Cognitive Decline From the Preclinical Stage: A Study of Post-stroke Small Vessel Disease. Front Neurol 12:687959. https://doi.org/10.3389/fneur.2021.687959
Goldwaser EL, Du X, Adhikari BM et al (2022) Role of white matter microstructure in impulsive behavior. JNP 34:254–260. https://doi.org/10.1176/appi.neuropsych.21070167
Pu W, Shen X, Huang M et al (2020) Assessment of white matter lesions in Parkinson’s disease: voxel-based analysis and tract-based spatial statistics analysis of Parkinson’s disease with mild cognitive impairment. Curr Neurovasc Res 17:480–486. https://doi.org/10.2174/1567202617666200901181842
Chen L, Zeng X, Zhou S, et al (2022) Correlation Between Serum High-Sensitivity C-Reactive Protein, Tumor Necrosis Factor-Alpha, Serum Interleukin-6 and White Matter Integrity Before and After the Treatment of Drug-Naïve Patients With Major Depressive Disorder. Front Neurosci 16:948637. https://doi.org/10.3389/fnins.2022.948637
Chen B, Akshita J, Han P et al (2020) Aberrancies of brain network structures in patients with anosmia. Brain Topogr 33:403–411. https://doi.org/10.1007/s10548-020-00769-2
Seppi K, Ray Chaudhuri K, Coelho M et al (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34:180–198. https://doi.org/10.1002/mds.27602
Gao C, Liu J, Tan Y, Chen S (2020) Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl Neurodegener 9:12. https://doi.org/10.1186/s40035-020-00191-5
Chen L, Hu X, Ouyang L et al (2016) A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 68:838–847. https://doi.org/10.1016/j.neubiorev.2016.07.022
Mander BA, Zhu AH, Lindquist JR et al (2017) White matter structure in older adults moderates the benefit of sleep spindles on motor memory consolidation. J Neurosci 37:11675–11687. https://doi.org/10.1523/JNEUROSCI.3033-16.2017
Rau Y-A, Wang S-M, Tournier J-D, et al (2019) A longitudinal fixel-based analysis of white matter alterations in patients with Parkinson’s disease. NeuroImage: Clinical 24:102098. https://doi.org/10.1016/j.nicl.2019.102098
Fernández-Miranda JC, Rhoton AL, Kakizawa Y et al (2008) The claustrum and its projection system in the human brain: a microsurgical and tractographic anatomical study: Laboratory investigation. JNS 108:764–774. https://doi.org/10.3171/JNS/2008/108/4/0764
Su W, Li K, Li C-M, et al (2021) Motor Symptom Lateralization Influences Cortico-Striatal Functional Connectivity in Parkinson’s Disease. Front Neurol 12:619631. https://doi.org/10.3389/fneur.2021.619631
Gotts SJ, Jo HJ, Wallace GL et al (2013) Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci USA 110:E3435-3444. https://doi.org/10.1073/pnas.1302581110
Di Tella S, Baglio F, Cabinio M et al (2018) Selection Processing in Noun and Verb Production in Left- and Right-Sided Parkinson’s Disease Patients. Front Psychol 9:1241. https://doi.org/10.3389/fpsyg.2018.01241
Foster PS, Drago V, Crucian GP et al (2011) Anxiety and depression severity are related to right but not left onset Parkinson’s disease duration. J Neurol Sci 305:131–135. https://doi.org/10.1016/j.jns.2011.02.023
Verreyt N, Nys GMS, Santens P, Vingerhoets G (2011) Cognitive differences between patients with left-sided and right-sided Parkinson’s disease. A Review. Neuropsychol Rev 21:405–424. https://doi.org/10.1007/s11065-011-9182-x
Miller IN, Cronin-Golomb A (2010) Gender differences in Parkinson’s disease: clinical characteristics and cognition: gender differences in Parkinson’s disease. Mov Disord 25:2695–2703. https://doi.org/10.1002/mds.23388
Bentivoglio AR, Lo Monaco MR, Liperoti R, et al (2021) Gender may be related to the side of the motor syndrome and cognition in idiopathic Parkinson’s disease. Neurologia (Engl Ed) 13:S0213–4853(21)00025–6. https://doi.org/10.1016/j.nrl.2021.01.009
Adwani S, Yadav R, Kumar K et al (2016) Neuropsychological profile in early Parkinson′s disease: comparison between patients with right side onset versus left side onset of motor symptoms. Ann Indian Acad Neurol 19:74. https://doi.org/10.4103/0972-2327.167711
Voruz P, Pierce J, Ahrweiller K et al (2022) Motor symptom asymmetry predicts non-motor outcome and quality of life following STN DBS in Parkinson’s disease. Sci Rep 12:3007. https://doi.org/10.1038/s41598-022-07026-5
Bove F, Cavallieri F, Castrioto A, et al (2022) Does Motor Symptoms Asymmetry Predict Motor Outcome of Subthalamic Deep Brain Stimulation in Parkinson’s Disease Patients? Front Hum Neurosci 16:931858. https://doi.org/10.3389/fnhum.2022.931858
Hershey T, Wu J, Weaver PM et al (2008) Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol 210:402–408. https://doi.org/10.1016/j.expneurol.2007.11.011
Acknowledgements
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative database (www.ppmi-info.org/data). For up-to-date information on the study, visit http://www.ppmi-info.org. PPMI, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including , including 4D Pharma; AbbVie Inc.; AcureX Therapeutics; Allergan; Amathus Therapeutics; Aligning Science Across Parkinson’s (ASAP); Avid Radiopharmaceuticals; Bial Biotech; Biogen; BioLegend; Bristol Myers Squibb; Calico Life Sciences LLC; Celgene Corporation; DaCapo Brainscience; Denali Therapeutics; the Edmond J. Safra Foundation; Eli Lilly and Company; GE Healthcare; GlaxoSmithKline; Golub Capital; Handl Therapeutics; Insitro; Janssen Pharmaceuticals; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; Neurocrine Biosciences; Pfizer Inc.; Piramal Imaging; Prevail Therapeutics; F. Hoffmann-La Roche Ltd and its affiliated company Genentech Inc.; Sanofi Genzyme; Servier; Takeda Pharmaceutical Company; Teva Neuroscience, Inc.; UCB, Vanqua Bio; Verily Life Sciences; Voyager Therapeutics, Inc.; and Yumanity Therapeutics, Inc (http://www.ppmi-info.org/fundingpartners).
Funding
This study was supported by National Natural Science Foundation of China (grant number: 82001367, receiver: Xi Liu), Natural Science Foundation of Chongqing (grant number: cstc2021jcyj-msxmX0180, receiver: Xi Liu), Kuanren Talent Program of The Second Affiliated Hospital of Chongqing Medical University (receiver: Xi Liu), and Scientific and Technological Research Program of Chongqing Municipal Education Commission (grant number: KJQN202200417, receiver: Changhong Tan).
Author information
Authors and Affiliations
Contributions
YZ: Data Curation (lead); Formal Analysis (lead); Investigation (lead); Methodology (lead); Software (lead); Validation (lead); Visualization (lead); Writing—Original Draft Preparation (equal). SL: Data Curation (supporting); Investigation (supporting); Validation (supporting). XD: Data Curation (supporting); Investigation (supporting); Validation (supporting). HL: Data Curation (supporting); Investigation (supporting); Validation (supporting). CT: Formal Analysis (supporting); Funding Acquisition (supporting). XL: Conceptualization (lead); Formal Analysis (supporting); Funding Acquisition (lead); Methodology (supporting); Software (supporting); Supervision (equal); Visualization (supporting); Writing—Original Draft Preparation (equal); Writing—Review & Editing (equal). FD: Conceptualization (supporting); Project Administration (equal); Resources (equal); Supervision (equal); Writing—Review & Editing (equal). LC: Project Administration (equal); Resources (equal); Supervision (equal). All authors read and approved the fnal manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The PPMI study has been approved by the respective institutional review boards of all participating sites, and written informed consents were obtained from each participant. This study was also approved by the Ethics Committee of The Second Affiliated Hospital of Chongqing Medical University (2022–759), written consents were also obtained from each participant.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Li, S., Da, X. et al. Study of the relationship between onset lateralization and hemispheric white matter asymmetry in Parkinson's disease. J Neurol 270, 5004–5016 (2023). https://doi.org/10.1007/s00415-023-11849-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11849-1