Abstract
Objectives
To apply a deep-learning algorithm to brain MRIs of seronegative patients with neuromyelitis optica spectrum disorders (NMOSD) and NMOSD-like manifestations and assess whether their structural features are similar to aquaporin-4-seropositive NMOSD or multiple sclerosis (MS) patients.
Patients and methods
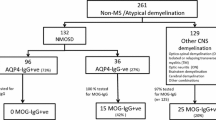
We analyzed 228 T2- and T1-weighted brain MRIs acquired from aquaporin-4-seropositive NMOSD (n = 85), MS (n = 95), aquaporin-4-seronegative NMOSD [n = 11, three with anti-myelin oligodendrocyte glycoprotein antibodies (MOG)], and aquaporin-4-seronegative patients with NMOSD-like manifestations (idiopathic recurrent optic neuritis and myelitis, n = 37), who were recruited from February 2010 to December 2019. Seventy-three percent of aquaporin-4-seronegative patients with NMOSD-like manifestations also had a clinical follow-up (median duration of 4 years). The deep-learning neural network architecture was based on four 3D convolutional layers. It was trained and validated on MRI scans of aquaporin-4-seropositive NMOSD and MS patients and was then applied to aquaporin-4-seronegative NMOSD and NMOSD-like manifestations. Assignment of unclassified aquaporin-4-seronegative patients was compared with their clinical follow-up.
Results
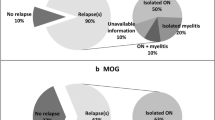
The final algorithm differentiated aquaporin-4-seropositive NMOSD and MS patients with an accuracy of 0.95. All aquaporin-4-seronegative NMOSD and 36/37 aquaporin-4-seronegative patients with NMOSD-like manifestations were classified as NMOSD. Anti-MOG patients had a similar probability of being NMOSD or MS. At clinical follow-up, one unclassified aquaporin-4-seronegative patient evolved to MS, three developed NMOSD, and the others did not change phenotype.
Conclusions
Our findings support the inclusion of aquaporin4-seronegative patients into NMOSD and suggest a possible expansion to aquaporin-4-seronegative unclassified patients with NMOSD-like manifestations. Anti-MOG patients are likely to have intermediate brain features between NMOSD and MS.


Similar content being viewed by others
Data availability
The dataset analyzed and the final algorithm are available on reasonable request.
References
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG (2015) NMOD international panel for, international consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85(2):177–189
Hamid SH, Elsone L, Mutch K, Solomon T, Jacob A (2017) The impact of 2015 neuromyelitis optica spectrum disorders criteria on diagnostic rates. Mult Scler 23(2):228–233
Hyun JW, Jeong IH, Joung A, Kim SH, Kim HJ (2016) Evaluation of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorder. Neurology 86(19):1772–1779
Ratelade J, Verkman AS (2012) Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol 44(9):1519–1530
Papadopoulos MC, Verkman AS (2012) Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11(6):535–544
Waschbisch A, Atiya M, Schaub C, Derfuss T, Schwab S, Lee DH, Muller M, Linker RA (2013) Aquaporin-4 antibody negative recurrent isolated optic neuritis: clinical evidence for disease heterogeneity. J Neurol Sci 331(1–2):72–75
Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, Kleiter I, Kleinschnitz C, Berthele A, Brettschneider J, Hellwig K, Hemmer B, Linker RA, Lauda F, Mayer CA, Tumani H, Melms A, Trebst C, Stangel M, Marziniak M, Hoffmann F, Schippling S, Faiss JH, Neuhaus O, Ettrich B, Zentner C, Guthke K, Hofstadt-van Oy U, Reuss R, Pellkofer H, Ziemann U, Kern P, Wandinger KP, Bergh FT, Boettcher T, Langel S, Liebetrau M, Rommer PS, Niehaus S, Munch C, Winkelmann A, Zettl UU, Metz I, Veauthier C, Sieb JP, Wilke C, Hartung HP, Aktas O, Paul F (2012) Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflamm 9:14
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B, Neuromyelitis Optica Study (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm 13(1):280
Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, Ciron J, Collongues N, Brassat D, Cotton F, Papeix C, Durand-Dubief F, Laplaud D, Deschamps R, Cohen M, Biotti D, Ayrignac X, Tilikete C, Thouvenot E, Brochet B, Dulau C, Moreau T, Tourbah A, Lebranchu P, Michel L, Lebrun-Frenay C, Montcuquet A, Mathey G, Debouverie M, Pelletier J, Labauge P, Derache N, Coustans M, Rollot F, De Seze J, Vukusic S, Marignier R, Ofsep, N.S. Group (2018) Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 90(21):e1858–e1869
Matthews L, Marasco R, Jenkinson M, Kuker W, Luppe S, Leite MI, Giorgio A, De Stefano N, Robertson N, Johansen-Berg H, Evangelou N, Palace J (2013) Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology 80(14):1330–1337
Cacciaguerra L, Meani A, Mesaros S, Radaelli M, Palace J, Dujmovic-Basuroski I, Pagani E, Martinelli V, Matthews L, Drulovic J, Leite MI, Comi G, Filippi M, Rocca MA (2019) Brain and cord imaging features in neuromyelitis optica spectrum disorders. Ann Neurol 85(3):371–384
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521(7553):436–444
McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, Tridandapani S, Auffermann WF (2018) Deep learning in radiology. Acad Radiol 25(11):1472–1480
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173
Tobin WO, Weinshenker BG, Lucchinetti CF (2014) Longitudinally extensive transverse myelitis. Curr Opin Neurol 27(3):279–289
Transverse Myelitis Consortium Working (2002) Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 59(4):499–505
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. In: proceedings of the IEEE conference on computer vision and pattern recognition, p 770-778
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, Huang Z, Karpathy A, Khosla A, Bernstein M, Berg AC (2015) ImageNet large scale visual recognition challenge. Int J Comp Vis 115(3):211–252
Hinton VNG (2010) Rectified linear units improve restricted Boltzmann machines. In: international conference on machine learning, Haifa, Israel
Gu HWX (2015) Max-pooling dropout for regularization of convolutional neural networks. In: F.U. Department of Electronic Engineering, Shanghai 200433, China
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15(1):1929–1958
Basha SS, Dubey SR, Pulabaigari V, Mukherjee S (2020) Impact of fully connected layers on the performance of convolutional neural networks for image classification. Neurocomputing 378:112–119
Lecun LBY, Bengio Y, Haffner P (1998) Gradient-based learning applied to document recognition. Proc IEEE 86(11):2278–2324
Kingma DP, Adam BJ (2015) A method for stochastic optimization. In: 3rd international conference for learning representations, San Diego, USA
Yuan LRY, Caponnetto A (2007) On early stopping in gradient descent learning. Constr Approx 26(2):289–315
Jurynczyk M, Tackley G, Kong Y, Geraldes R, Matthews L, Woodhall M, Waters P, Kuker W, Craner M, Weir A, DeLuca GC, Kremer S, Leite MI, Vincent A, Jacob A, de Seze J, Palace J (2017) Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J Neurol Neurosurg Psychiatry 88(2):132–136
Solomon AJ, Bourdette DN, Cross AH, Applebee A, Skidd PM, Howard DB, Spain RI, Cameron MH, Kim E, Mass MK, Yadav V, Whitham RH, Longbrake EE, Naismith RT, Wu GF, Parks BJ, Wingerchuk DM, Rabin BL, Toledano M, Tobin WO, Kantarci OH, Carter JL, Keegan BM, Weinshenker BG (2016) The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology 87(13):1393–1399
Eshaghi A, Wottschel V, Cortese R, Calabrese M, Sahraian MA, Thompson AJ, Alexander DC, Ciccarelli O (2016) Gray matter MRI differentiates neuromyelitis optica from multiple sclerosis using random forest. Neurology 87(23):2463–2470
Do Rego CA, Collongues N (2018) Neuromyelitis optica spectrum disorders: features of aquaporin-4, myelin oligodendrocyte glycoprotein and double-seronegative-mediated subtypes. Revue Neurol 174(6):458–470
Wei Y, Chang H, Li X, Du L, Xu W, Cong H, Yao Y, Zhang X, Yin L (2018) CSF-S100B is a potential candidate biomarker for neuromyelitis optica spectrum disorders. Biomed Res Int 2018:5381239
Mealy MA, Kim SH, Schmidt F, Lopez R, Jimenez Arango JA, Paul F, Wingerchuk DM, Greenberg BM, Kim HJ, Levy M (2018) Aquaporin-4 serostatus does not predict response to immunotherapy in neuromyelitis optica spectrum disorders. Mult Scler 24(13):1737–1742
Wang X, Chen X, Zhu C, Ma H, Wang F, Qin L, Li W (2018) A multi-facet comparative analysis of neuromyelitis optica spectrum disorders in patients with seropositive and seronegative AQP4-IgG. Medicine 97(48):e13100
Sun J, Sun X, Zhang N, Wang Q, Cai H, Qi Y, Li T, Qin W, Yu C (2017) Analysis of brain and spinal cord lesions to occult brain damage in seropositive and seronegative neuromyelitis optica. Eur J Radiol 94:25–30
Petzold A, Woodhall M, Khaleeli Z, Tobin WO, Pittock SJ, Weinshenker BG, Vincent A, Waters P, Plant GT (2019) Aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies in immune-mediated optic neuritis at long-term follow-up. J Neurol Neurosurg Psychiatry 90(9):1021–1026
Jiao Y, Fryer JP, Lennon VA, McKeon A, Jenkins SM, Smith CY, Quek AM, Weinshenker BG, Wingerchuk DM, Shuster EA, Lucchinetti CF, Pittock SJ (2014) Aquaporin 4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. JAMA Neurol 71(1):48–54
Alvarenga MP, Alvarenga RM, Alvarenga MP, Santos AM, Thuler LC (2012) Anti-AQP(4) antibody in idiopathic acute transverse myelitis with recurrent clinical course: frequency of positivity and influence in prognosis. J Spinal Cord Med 35(4):251–255
Deschamps R, Gueguen A, Lecler A, Lecouturier K, Lamirel C, Bensa C, Marignier R, Vignal C, Gout O (2018) Acute idiopathic optic neuritis: not always benign. Eur J Neurol 25(11):1378–1383
Carnero Contentti E, Hryb JP, Morales S, Gomez A, Chiganer E, Di Pace JL, Lessa C, Perassolo M (2017) Longitudinally extensive transverse myelitis immune-mediated in aquaporin-4 antibody negative patients: disease heterogeneity. J Neurol Sci 373:134–137
Takahashi-Fujigasaki J, Takagi S, Sakamoto T, Inoue K (2009) Spinal cord biopsy findings of anti-aquaporin-4 antibody-negative recurrent longitudinal myelitis in a patient with sicca symptoms and hepatitis C viral infection. Neuropathology 29(4):472–479
Castelvecchi D (2016) Can we open the black box of AI? Nature 538(7623):20–23
Funding
None.
Author information
Authors and Affiliations
Contributions
LC: data analysis, statistical analysis, drafting/revising the manuscript. LS: data analysis, statistical analysis, drafting/revising the manuscript. MR: patient recruitment, clinical assessment, data analysis. SM: patient recruitment, clinical assessment, data analysis. LM: patient recruitment, clinical assessment, data analysis. JD: patient recruitment, clinical assessment, data analysis. MF: study concept, drafting/revising the manuscript. MAR: study concept, drafting/revising the manuscript, MRI data analysis.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics committee approval
Approval was received from the local ethical standards committee (IRCCS San Raffaele Scientific Institute) on human experimentation. The study conforms to the Declaration of Helsinki.
Consent to participate
All subjects signed a written informed consent before study participation.
Rights and permissions
About this article
Cite this article
Cacciaguerra, L., Storelli, L., Radaelli, M. et al. Application of deep-learning to the seronegative side of the NMO spectrum. J Neurol 269, 1546–1556 (2022). https://doi.org/10.1007/s00415-021-10727-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10727-y