Abstract
Objective
To compare the available diagnostic criteria for progressive multifocal leukoencephalopathy (PML) diagnosis in a real-world cohort of patients with natalizumab-associated PML and to explore opportunities for improvement of such criteria in the context of pharmacovigilance of immunosuppressive therapies.
Methods
We applied the “Mentzers PML case definition” to a dataset of 28 patients with natalizumab-associated PML (many of whom were identified through MRI screening in the context of pharmacovigilance), who were previously rated according to the American Academy of Neurology (AAN) PML diagnostic criteria, and compared the response to both sets of criteria.
Results
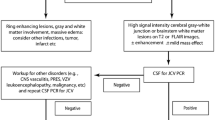
The “Mentzers case definition” resulted in a level of certainty 1–3 in patients with a positive JC virus PCR, termed ‘definite’ and ‘probable’ PML according to the AAN diagnostic criteria. Patients that tested negative for JC virus in CSF (29%) were classified level 4 by the “Mentzers case definition”, neglecting the longitudinal clinical and radiological signs of PML available, while the AAN diagnostic criteria separated these patients in ‘possible’ and ‘not PML’.
Conclusions
Both the AAN PML diagnostic criteria and the “Mentzers case definition” require the positive detection of JC virus DNA in CSF to define patients at a higher degree of suspicion of PML. However, as sensitivity of JC virus PCR in CSF is limited and often returns negative in particular in early cases of PML with a mere MRI-based PML suspicion, both criteria have obvious limitations when frequent MRI is used for pharmacovigilance purposes. Thus, revision of PML diagnostic criteria is needed, including the incorporation of lesion evolution, and longitudinal CSF studies that also assess for the presences of intrathecally produced anti-JC virus antibodies.
Similar content being viewed by others
References
Major EO, Yousry TA, Clifford DB (2018) Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol 17:467–480
Berger JR, Aksamit AJ, Clifford DB et al (2013) PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 80:1430–1438
Wijburg MT, Warnke C, Barkhof F, Uitdehaag BMJ, Killestein J, Wattjes MP (2019) Performance of PML diagnostic criteria in natalizumab-associated PML: data from the Dutch-Belgian cohort. J Neurol Neurosurg Psychiatry 90:44–46
Mentzer D, Prestel J, Adams O et al (2012) Case definition for progressive multifocal leukoencephalopathy following treatment with monoclonal antibodies. J Neurol Neurosurg Psychiatry 83:927–933
Wijburg MT, Kleerekooper I, Lissenberg-Witte BI et al (2018) Association of progressive multifocal leukoencephalopathy lesion volume with JC virus polymerase chain reaction results in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. JAMA Neurol 75:827–833
Blankenbach K, Schwab N, Hofner B, Adams O, Keller-Stanislawski B, Warnke C (2019) Natalizumab-associated progressive multifocal leukoencephalopathy in Germany. Neurology 92:e2232–2239
Wattjes MP, Wijburg MT, van Eijk J et al (2018) Inflammatory natalizumab-associated PML: baseline characteristics, lesion evolution and relation with PML-IRIS. J Neurol Neurosurg Psychiatry 89:535–541
Warnke C, Wijburg MT, Hartung HP, Killestein J, Adams O, Wattjes MP (2017) Application of the CSF JCV antibody index to early natalizumab-associated progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry 88:1092–1094
Warnke C, von Geldern G, Markwerth P et al (2014) Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 76:792–801
Dalla Costa G, Martinelli V, Moiola L et al (2019) Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab-treated multiple sclerosis patients. Ann Neurol 85:606–610
Loonstra FC, Verberk IMW, Wijburg MT et al (2019) Serum neurofilaments as candidate biomarkers of natalizumab associated progressive multifocal leukoencephalopathy. Ann Neurol 86:322–324
Acknowledgements
The authors wish to thank all patients included in the study for agreeing to the use of their MR images and paraclinical data for research and education purposes. In addition, we would like to thank the following physicians for sharing clinical, imaging and paraclinical data on PML patients included in this study: Bob W van Oosten and Chris H Polman (VU University Medical Center, Amsterdam, The Netherlands), Dorine A Siepman and Rogier Hintzen (Erasmus MC, University Medical Center Rotterdam, The Netherlands), Jop Mostert (Rijnstate Hospital, Department of Neurology, Arnhem, The Netherlands), Wibe Moll (Maasstad Hospital, Rotterdam, The Netherlands), Alex EL van Golde (ZGT Hospital, Almelo, The Netherlands), Stephan TFM Frequin (St Antonius Hospital, Nieuwegein, The Netherlands), Paul AD Bouma (Tergooi, Blaricum, Hilversum, The Netherlands), Cristina Tiu (Bucharest, Romania), and Bénédicte Quivron (CH Jolimont, La Louvière, Belgium), Jean Braeckeveldt (Epicura, Baudour, Belgium), Erik van Munster and Jeroen van Eijk (Department of Neurology, Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch, The Netherlands), Thea Heersema (Department of Neurology, University Medical Center Groningen, Groningen, The Netherlands), Jaap de Graaf (Isala Hospital, Zwolle, The Netherlands), and Raymond MM Hupperts (Zuyderland Medical Center, Sittard, The Netherlands).
Funding
The MS Center Amsterdam is funded by a program grant (14-358e) from the Stichting voor MS Research (Voorschoten, The Netherlands). CW received support from the Hertie foundation (P1150063) for this work. None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization and design of the study: MTW, CW, JK, and MPW. Acquisition and analysis of data: MTW, JK, and MPW. Drafting of a significant portion of the manuscript and table: MTW, CW, JK, and MPW.
Corresponding author
Ethics declarations
Conflicts of interest
MTW does not report any competing interest. CW has received speaking fees (institutional only) or research support from Novartis, Biogen, Roche and Sanofi Genzyme. JK has received consultancy fees from Merck-Serono, Teva, Biogen, Genzyme and Novartis. MPW has received speaker or consultancy fees from Bayer, Biogen, Biologix, Celgene, Genilac, Imcyse, IXICO, Medison, Merck-Serono, Novartis, Sanofi Genzyme, Roche, Teva, UCB Pharma.
Code availability
Software application.
Ethical approval
We obtained a waiver from our local institutional review board stating that the requirements of the Medical Research Involving Human Subjects Act did not apply and that official IRB approval was not mandatory. Written informed consent was obtained from all participants for the use of the clinical, laboratory and imaging data for research and teaching purposes.
Informed consent
Written informed consent was obtained from all participants for the use of the clinical, laboratory and imaging data for research and teaching purposes. Written informed consent was obtained from all participants for publishing the data.
Rights and permissions
About this article
Cite this article
Wijburg, M.T., Warnke, C., Killestein, J. et al. Application of “Mentzer’s PML case definition” to natalizumab-treated patients in the setting of strict MRI-based pharmacovigilance. J Neurol 267, 2599–2602 (2020). https://doi.org/10.1007/s00415-020-09880-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09880-7