Abstract
Background
It has been described that treating relapsing–remitting multiple sclerosis (RRMS) patients with alemtuzumab following fingolimod could be less effective due to the different dynamics of lymphocyte repopulation. Effectiveness and safety of alemtuzumab compared to rituximab after fingolimod withdrawal were analyzed.
Patients and methods
A follow-up of a cohort of RRMS patients treated with alemtuzumab or rituximab after fingolimod withdrawal was accomplished. Effectiveness, measured by the percentage of patients with no evidence of disease activity (NEDA), and the presence of side effects (SE) were registered.
Results
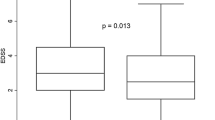
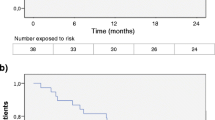
Fifty-five patients, 28 with alemtuzumab and 27 with rituximab, were analyzed. No differences in the washout period or in the baseline lymphocytes counts were observed. After a mean follow-up period of 28.8 months, the annualized relapsing rate was significantly reduced in the alemtuzumab group from 1.29 to 0.004 (p < 0.001) and in the rituximab group from 1.24 to 0.02 (p < 0.001), without differences. A significant reduction of the median EDSS from 2.8 to 2.0 in the alemtuzumab group and from 3.5 to 2.5 (p < 0.01) in the rituximab group was observed, without differences. Eighty-two per cent (n = 28) of patients in alemtuzumab group and 69.2% (n = 26) in rituximab group achieved NEDA criteria, without differences (p = 0.3). Symptoms related to the infusion were the most frequent SE in both groups. No serious SE were registered.
Conclusion
Treating RRMS patients with alemtuzumab or rituximab after fingolimod withdrawal is effective and safe, without significant differences between both groups in our series.


Similar content being viewed by others
References
Duddy M, Bar-Or A (2006) B-cells in multiple sclerosis. Int MS J 13:84–90
Lucchinetti C, Brück W, Parisi J et al (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Faissner S, Gold R (2018) Efficacy and safety of the newer multiple sclerosis drugs approved since 2010. CNS Drugs 32:269–287. https://doi.org/10.1007/s40263-018-0488-6
Merkel B, Butzkueven H, Traboulsee AL et al (2017) Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev 16:658–665. https://doi.org/10.1016/j.autrev.2017.04.010
Blinkenberg M, Sørensen PS (2017) Monoclonal antibodies for relapsing multiple sclerosis: a review of recently marketed and late-stage agents. CNS Drugs 31:357–371. https://doi.org/10.1007/s40263-017-0414-3
Rommer PS, Zettl UK (2018) Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 19:483–498. https://doi.org/10.1080/14656566.2018.1446944
Sedal L, Winkel A, Laing J et al (2017) Current concepts in multiple sclerosis therapy. Degener Neurol Neuromuscul Dis 7:109–125. https://doi.org/10.2147/DNND.S109251
Gholamzad M, Ebtekar M, Ardestani MS et al (2018) A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res. https://doi.org/10.1007/s00011-018-1185-0
Wingerchuk DM, Weinshenker BG (2016) Disease modifying therapies for relapsing multiple sclerosis. BMJ 354:i3518
Pardo G, Jones DE (2017) The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol 264:2351–2374. https://doi.org/10.1007/s00415-017-8594-9
Grand F, Maison F, Yeung M, Morrow S et al (2018) Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches. Neural Regen Res 13:1871. https://doi.org/10.4103/1673-5374.239432
Khatri B, Barkhof F, Comi G et al (2011) Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 10:520–529. https://doi.org/10.1016/S1474-4422(11)70099-0
Bianco A, Patanella AK, Nociti V et al (2014) Second-line therapy with fingolimod for relapsing-remitting multiple sclerosis in clinical practice: the effect of previous exposure to natalizumab. Eur Neurol 73:57–65. https://doi.org/10.1159/000365968
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415. https://doi.org/10.1056/NEJMoa0907839
Hersh CM, Hara-Cleaver C, Rudick RA et al (2014) Experience with fingolimod in clinical practice. Int J Neurosci. https://doi.org/10.3109/00207454.2014.969839
Ayzenberg I, Hoepner R, Kleiter I (2016) Fingolimod for multiple sclerosis and emerging indications: appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag 12:261–272. https://doi.org/10.2147/TCRM.S65558
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839. https://doi.org/10.1016/S0140-6736(12)61768-1
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380:1819–1828. https://doi.org/10.1016/S0140-6736(12)61769-3
Havrdova E, Arnold DL, Cohen JA et al (2017) Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 89:1107–1116. https://doi.org/10.1212/WNL.0000000000004313
Ziemssen T, Thomas K (2017) Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 10:343–359. https://doi.org/10.1177/1756285617722706
Brown JWL, Coles AJ (2013) Alemtuzumab: evidence for its potential in relapsing-remitting multiple sclerosis. Drug Des Dev Ther 7:131–138. https://doi.org/10.2147/DDDT.S32687
Castillo-Trivino T, Braithwaite D, Bacchetti P, Waubant E (2013) Rituximab in relapsing and progressive forms of multiple sclerosis: a systematic review. PLoS One 8:e66308. https://doi.org/10.1371/journal.pone.0066308
Barra ME, Soni D, Vo KH et al (2016) Experience with long-term rituximab use in a multiple sclerosis clinic. Mult Scler J Exp Transl Clin. https://doi.org/10.1177/2055217316672100
Alcalá C, Gascón F, Pérez-Miralles F et al (2018) Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol 265:1690–1697. https://doi.org/10.1007/s00415-018-8899-3
Willis M, Pearson O, Illes Z et al (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4:e320. https://doi.org/10.1212/NXI.0000000000000320
Huhn K, Bayas A, Doerck S et al (2018) Alemtuzumab as rescue therapy in a cohort of 50 relapsing–remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265:1521–1527. https://doi.org/10.1007/s00415-018-8871-2
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Coles AJ, Compston DAS, Selmaj KW et al (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359:1786–1801. https://doi.org/10.1056/NEJMoa0802670
Coles AJ, Cohen JA, Fox EJ et al (2017) Alemtuzumab CARE-MS II 5-year follow-up. Neurology 89:1117–1126. https://doi.org/10.1212/WNL.0000000000004354
Vidal-Jordana A, Tintoré M, Tur C et al (2015) Significant clinical worsening after natalizumab withdrawal: predictive factors. Mult Scler 21:780–785. https://doi.org/10.1177/1352458514549401
Miravalle A, Jensen R, Kinkel RP (2011) Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 68:186–191. https://doi.org/10.1001/archneurol.2010.257
Havla J, Gerdes LA, Meinl I et al (2011) De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669. https://doi.org/10.1007/s00415-011-5996-y
Ghadiri M, Fitz-Gerald L, Rezk A et al (2017) Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. Mult Scler J 23:1225–1232. https://doi.org/10.1177/1352458517713147
Sánchez P, Meca-Lallana V, Vivancos J (2018) Tumefactive multiple sclerosis lesions associated with fingolimod treatment: report of 5 cases. Mult Scler Relat Disord 25:95–98. https://doi.org/10.1016/j.msard.2018.07.001
Frau J, Sormani MP, Signori A et al (2018) Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur J Neurol 25:1270–1275. https://doi.org/10.1111/ene.13694
Holmøy T, Torkildsen Ø, Zarnovicky S (2018) Extensive multiple sclerosis reactivation after switching from fingolimod to rituximab. Case Rep Neurol Med 2018:1–3. https://doi.org/10.1155/2018/5190794
Mills EA, Mao-Draayer Y (2018) Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult Scler J 24:1014–1022. https://doi.org/10.1177/1352458518775550
Guarnera C, Bramanti P, Mazzon E (2017) Alemtuzumab: a review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther Clin Risk Manag 13:871–879. https://doi.org/10.2147/TCRM.S134398
Šega-Jazbec S, Barun B, Horvat Ledinek A et al (2017) Management of infusion related reactions associated with alemtuzumab in patients with multiple sclerosis. Mult Scler Relat Disord 17:151–153. https://doi.org/10.1016/j.msard.2017.07.019
Rotondi M, Molteni M, Leporati P et al (2017) Autoimmune thyroid diseases in patients treated with alemtuzumab for multiple sclerosis: an example of selective anti-TSH-receptor immune response. Front Endocrinol (Lausanne) 8:254. https://doi.org/10.3389/fendo.2017.00254
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
All patients included in the study acceded to donate and signed a specific informed consent and all research was conducted following legal and ethical requirements at the Research Institute of the Hospital La Fe and was approved by its Institutional Review Board.
Rights and permissions
About this article
Cite this article
Alcalá, C., Gascón, F., Pérez-Miralles, F. et al. Treatment with alemtuzumab or rituximab after fingolimod withdrawal in relapsing–remitting multiple sclerosis is effective and safe. J Neurol 266, 726–734 (2019). https://doi.org/10.1007/s00415-019-09195-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09195-2