Abstract
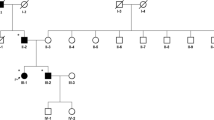
We studied the presynaptic nigrostriatal dopaminergic function using single photon emission computed tomography (SPECT) imaging of a 99mTc-TRODAT-1 (TRODAT) scan in a dopa-responsive dystonia (DRD) family with the guanosine triphosphate cyclohydrolase 1 (GCH-1) gene mutation. Clinically, there was presentation of intrafamilial variability in the DRD family. The index patient was a 10-year-old girl with classic DRD and normal presynaptic nigrostriatal dopaminergic function. However, her grandmother, a 79-year-old woman, presented with slowly progressive Parkinson’s disease (PD) without dystonic symptoms and excellent response to dopaminergic therapy for 21 years. Her brain TRODAT SPECT imaging revealed a markedly and asymmetrically reduced uptake of dopamine transporter at the bilateral striatum. Her father, a 54-year-old man, was an asymptomatic gene carrier and his brain TRODAT SPECT imaging revealed asymmetrically reduced nigrostriatal dopaminergic transmission in the bilateral striatum. We conclude variability of presynaptic nigrostriatal dopaminergic function in patients with DRD is related to their clinical heterogeneity. Significantly, impairment of presynaptic dopamine function actually occurs in the asymptomatic gene carrier.


Similar content being viewed by others
References
Wijemannes S, Jankovic J (2015) Dopa-responsive dystonia-clinical and genetic heterogeneity. Nat Rev Neurol 7:414–424
Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Yanaka H, Tsuji S et al (1994) Hereditary progressive dystonia with marked diurnal fluctuation caused by mutation in the GTP cyclohydrolase I gene. Nat Genet 8:236–242
Hanihara T, Inoue K, Kawanishi C, Owado M (1997) 6-pyruvoyl-tetrahydropterin synthase deficiency with generalized dystonia and diurnal fluctuation of symptoms: a clinical and molecular studies. Mov Disord 12:408–411
Wilder-Smith E, Tan EK, Law HY, Zhao Y, Ng I, Wong MC (2003) Spinocerebellar ataxia type 3 presenting as an L-DOPA responsive dystonia phenotype in a Chinese family. J Neurol Sci 231:25–28
Clarlesworth G, Mohire MD, Schneider SA, Stamelou M, Wood NW, Bhatia JP (2013) Ataxia telangiectasia presenting a dopa-responsive cervical dystonia. Neurology 81:1148–1151
Wijemanne S, Shulman JM, Jimenez-Shahed J, Curry D, Jankovic J (2015) SP11 mutations associated with a complex phenotype resembling dopa-responsive dystonia. Mov Disord Clin Pract 2:149–154
Segawa M, Nomura Y, Nishiyama N (2003) Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease). Ann Neurol 54:532–545
Trender-Gerhard I, Sweeney MG, Schwingenschuh P, Mir P, Edwards MJ, Gerhard A, Polke JM, Hanna MG, Davis MB, Wood NW, Bhatia KP (2009) Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry 80:839–845
Segawa M, Ohmi K, Ito S, Aoyama M, Haykawa H (1971) Childhood basal ganglia disease with remarkable response to L-DOPA, hereditary basal ganglia disease with marked diurnal fluctuation. Shinryo (Tokyo) 24:667–672 (in Japanese)
Turjanski N, Bhatia K, Burn DJ, Sawle GV, Marsden CD, Brooks DJ (1993) Comparison of striatal 18F-dopa uptake in adult-onset dystonia-parkinsonism, Parkinson’s disease, and dopa-responsive dystonia. Neurology 43:1563–1568
Naumann M, Pirker W, Reiners K, Lange KW, Becker G, Brucke T (1997) [123I]-beta-CIT single photon emission tomography in dopa-responsive dystonia. Mov Disord 12:448–451
Jeon BS, Jeong JM, Park SS, Kim JM, Chang YS, Song HC, Kim KM, Yoon KY, Lee MC, Lee SB (1998) Dopamine transporter density measured by [123I]-B-CIT single-photon emission computed tomography is normal in dopa-responsive dystonia. Ann Neurol 43:792–800
Huang CC, Yen YC, Weng YS, Lu CS (2002) Normal dopamine transporter binding in dopa-responsive dystonia. J Neurol 249:1016–1020
Kikuchi A, Takeda A, Fujihara K, Shiga Y, Tanji H, Nagai M, Ichinose H, Okamua N, Arai H, Itoyama Y (2004) Arg(184)His mutant GTP cyclohydrolase I, causing recessive hyperphenylalaninemia, is responsible for dopa-responsive dystonia with Parkinsonism: a case report. Mov Disord 19:590–593
Hjermind LE, Johannsen LG, Blau N, Wevers RA, Lucking CB, Hertz JM, Friberg L, Regeur L, Nielsen JE, Sorensen SA (2006) Dopa-responsive dystonia and early-onset Parkinson’s disease in a patient with GTP cyclohydrolase I deficiency? Mov Disord 21:679–713
Eggers C, Volk AE, Kahraman D, Fink GR, Leube B, Schmidt M, Timmermann L (2012) Are dopa-responsive dystonia and Parkinson’s disease related disorders? A case report. Parksonism Rel Disord 18:666–668
Mencacci NE, Isaias IU, Reich MM, Ganos C, Plagnol V, Polke JM et al (2014) Parkinson’s disease in GTP cyclohydrolase 1 mutation carrier. Brain 137:2480–2492
Lewthwaite AJ, Lambert TD, Polfe EB, Olgiati S, Quadri M, Simons EJ, Morrison KE, Bonifati V, Nichol DJ (2015) Novel CGH1 variant in dopa-responsive dystonia and Parkinson’s disease. Parkinsonism Rel Disord 21:394–397
Huang WS, Lin SZ, Lin JC, Wey SP, Tin G, Lu RS (2001) Evaluation of early-stage Parkinson’s disease with 99mTc-TRODAT-1 imaging. J Nucl Med 42:1303–1308
Wu-Chou YH, Yeh TH, Wang CY, Lin JJ, Huang CC, Chang SC, Lai SC, Chen RS, Weng YS, Huang CL, Lu CS (2010) High frequency of multiexonic deletion of the GCH1 gene in a Taiwanese cohort of dopa-responsive dystonia. Am J Genet Part B 153B:903–908
Zirn B, Steinberger D, Troidl C, Brockmann K, von der Hagen M, Feiner C, Henke L, Müller U, Wang HC, Cheng SJ (2008) Frequency of GCH1 deletion in dopa-responsive dystonia. J Neurol Neurosurg Psychiatry 79:183–186
Wide C, Melquist S, Hauf M, Solida A, Cobb SA, Kachergus JM et al (2008) Study of a Swiss dopa-responsive dystonia family with a deletion in GCH1 redefining DTY14 as DTY5. Neurology 70:1377–1383
Momma K, Funayama M, Li Y, Ichinose H, Motoyoshi K, Hattori N, Mizuno Y, Kamakura K (2009) A new mutation in the GCH-1 gene presents as early-onset parkinsonism. Parksonism Rel Disord 15:160–161
Socal MP, Emmel VE, Rieder CR, Hilbig A, Saraiva-Pereira ML, Jardim LB (2009) Intrafamilial variability of Parkinson phenotype in SCA2: novel cases due to SCA2 and SCA3 expansions. Parkinsonism Rel disord 15:374–378
De Souza PV, de Rezende Pinto WB, de Rezende Batistella GN, Bortholin T, Oliveira AS (2017) Hereditary spastic paraplegia: clinical and genetic hallmarks. Cerebellum 16:525–551
Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M (2016) Adrenoleukodystrophy-neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol 12:606–615
Brooks DJ (2012) Parkinson’s disease: diagnosis. Parkinsonism Rel Disord 18(suppl 1):S31–S33
Lu CS, Wu Chou YH, Yen TZ, Tsai CH, Cehn RS, Chang HC (2002) Dopa-responsive parkinsonian phenotype of spinocerebellar ataxia type 2. Mov Disord 17:1046–1051
Lu CS, Chang HC, Kuo PC, Liu YL, Wu WS, Weng YS, Yen TZ, Wu Chou YH (2004) The parkinsonian phenotype of spinocerebellar ataxia type 3 in a Taiwanese family. Parkinonism Rel Disord 10:369–373
Yen TC, Tzen KY, Chen MC, Wu Chou YH, Chen RS, Wey SP, Ting G, Lu CS (2002) Dopamine transporter concentration is reduced in asymptomatic Machado-Joseph disease gene carriers. J Nucl Med 43:153–159
Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Chen H, Zhao E, Zhang W, Yu Y, Liu R, Huang X, Ciesielski-Jones AJ, Justice MA, Cousins DS, Peddada S (2015) Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Trans Neurodegener 4:1
Booij J, Knol RJJ (2007) SPECT imaging of the dopaminergic system in (premotor) Parkinson’s disease. Parkinsonism Rel Disord (suppl 3):S425–S428
Berendse HW, Ponsen MM (2009) Diagnosing premotor Parkinson’s disease using a two-step approach combining olfactory testing and DAT SPECT imaging. Parkinsonism Rel Disord 15(Suppl 3):S26–S30
Ponsen MM, Stoffers D, Ech Wolters, Booij Berendse HW (2010) Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:396–399
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, JJ., Lu, CS. & Tsai, CH. Variability of presynaptic nigrostriatal dopaminergic function and clinical heterogeneity in a dopa-responsive dystonia family with GCH-1 gene mutation. J Neurol 265, 478–485 (2018). https://doi.org/10.1007/s00415-017-8723-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8723-5