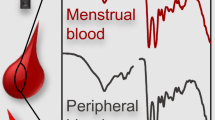
Abstract
Body fluids are one of the most important pieces of evidence encountered in forensic cases especially in cases of sexual assault. Analysis of such evidence can help to establish a link between the perpetrator, the victim, and the crime scene and thereby assist in crime reconstruction. However, one of the biggest challenges faced by the investigators in sexual assault cases is that of ascertaining the issue of consent of the victim. In this matter, differentiation of menstrual blood (either in dried or stained form) from traumatic peripheral blood can give a potential solution on this particular aspect. A number of studies have been attempted to differentiate these two body fluids using various biochemical and serological methods. However, the methods employed are limited by factors such as sample destructivity and non-specificity, and the methods are susceptible to false positive results. In the present study, the scope of attenuated total reflectance (ATR)-Fourier transform infrared (FT-IR) spectroscopy in discriminating samples of menstrual blood and peripheral blood has been investigated, in combination with chemometric tools such as principal component analysis (PCA), partial least square regression (PLSR), and linear discriminant analysis (LDA). PCA resulted in 93.3% accuracy, whereas PLSR and LDA resulted in 100% accuracy for the discrimination of peripheral blood from menstrual blood. Application of PCA for the discrimination of menstrual blood from vaginal fluid and seminal fluid delivered 100% classification. Similarly, 100% classification was achieved while differentiating between menstrual blood and blood look-alike substances. Furthermore, in the current study, the effect of substrates on the analysis of menstrual blood has also been studied and described.

Graphical Abstract













Similar content being viewed by others
References
Virkler K, Lednev IK (2009) Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int 188:1–17. https://doi.org/10.1016/j.forsciint.2009.02.013
An JH, Shin KJ, Yang WI, Lee HY (2012) Body fluid identification in forensics. BMB Rep 45:545–553. https://doi.org/10.5483/BMBRep.2012.45.10.206
Holtkötter H, Dias Filho CR, Schwender K, Stadler C, Vennemann M, Pacheco AC, Roca G (2018) Forensic differentiation between peripheral and menstrual blood in cases of alleged sexual assault—validating an immunochromatographic multiplex assay for simultaneous detection of human hemoglobin and D-dimer. Int J Legal Med 132:683–690. https://doi.org/10.1007/s00414-017-1719-y
Baker DJ, Grimes EA, Hopwood AJ (2011) D-dimer assays for the identification of menstrual blood. Forensic Sci Int 212:210–214. https://doi.org/10.1016/j.forsciint.2011.06.013
Jakubowska J, Maciejewska A, Bielawski KP, Pawłowski R (2014) mRNA heptaplex protocol for distinguishing between menstrual and peripheral blood. Forensic Sci Int Genet 13:53–60. https://doi.org/10.1016/j.fsigen.2014.07.006
Gray D, Frascione N, Daniel B (2012) Development of an immunoassay for the differentiation of menstrual blood from peripheral blood. Forensic Sci Int 220:12–18. https://doi.org/10.1016/j.forsciint.2012.01.020
Juusola J, Ballantyne J (2007) mRNA profiling for body fluid identification by multiplex quantitative RT-PCR. J Forensic Sci 52:1252–1262. https://doi.org/10.1111/j.1556-4029.2007.00550.x
Jakubowska J, Maciejewska A, Pawłowski R, Bielawski KP (2013) mRNA profiling for vaginal fluid and menstrual blood identification. Forensic Sci Int Genet 7:272–278. https://doi.org/10.1016/j.fsigen.2012.11.005
Hanson EK, Mirza M, Rekab K, Ballantyne J (2014) The identification of menstrual blood in forensic samples by logistic regression modeling of miRNA expression. Electrophoresis 35:3087–3095. https://doi.org/10.1002/elps.201400171
Choi A, Shin KJ, Yang WI, Lee HY (2014) Body fluid identification by integrated analysis of DNA methylation and body fluid-specific microbial DNA. Int J Legal Med 128:33–41. https://doi.org/10.1007/s00414-013-0918-4
Hanson EK, Ballantyne J (2013) Rapid and inexpensive body fluid identification by RNA profiling-based multiplex high resolution melt (HRM) analysis [version 1; peer review: 2 approved]. F1000Research 2. https://doi.org/10.12688/f1000research.2-281.v1
Yang H, Zhou B, Prinz M, Siegel D (2012) Proteomic analysis of menstrual blood. Mol Cell Proteomics 11:1024 LP–1021035. https://doi.org/10.1074/mcp.M112.018390
Whitehead PH, Divall GB (1974) The identification of menstrual blood — the immunoelectrophoretic characterisation of soluble fibrinogen from menstrual bloodstain extracts. Forensic Sci 4:53–62. https://doi.org/10.1016/0300-9432(74)90076-4
Akutsu T, Watanabe K, Motani H, Iwase H, Sakurada K (2012) Evaluation of latex agglutination tests for fibrin–fibrinogen degradation products in the forensic identification of menstrual blood. Legal Med 14:51–54. https://doi.org/10.1016/j.legalmed.2011.10.003
Whitehead PH, Divall GB (1973) Assay of “soluble fibrinogen” in bloodstain extracts as an aid to identification of menstrual blood in forensic science: preliminary findings. Clin Chem 19:762 LP–762765
Divall GB, Ismail M (1983) Lactate dehydrogenase isozymes in vaginal swab extracts: a problem for the identification of menstrual blood. Forensic Sci Int 21:139–147. https://doi.org/10.1016/0379-0738(83)90102-0
Bauer M, Patzelt D (2008) Identification of menstrual blood by real time RT-PCR: technical improvements and the practical value of negative test results. Forensic Sci Int 174:55–59. https://doi.org/10.1016/j.forsciint.2007.03.016
Ota S, Furuya Y, Fujii K (1965) Identification of menstrual blood stains--experimental studies on the detection of glycogen of vaginal epithelial cells. Nihon Hoigaku Zasshi 19:300–305
Haas C, Klesser B, Maake C, Bär W, Kratzer A (2009) mRNA profiling for body fluid identification by reverse transcription endpoint PCR and realtime PCR. Forensic Sci Int Genet 3:80–88. https://doi.org/10.1016/j.fsigen.2008.11.003
Salamonsen LA, Kovacs GT, Findlay JK (1999) Current concepts of the mechanisms of menstruation. Best Pract Res Clin Obstet Gynaecol 13:161–179. https://doi.org/10.1053/beog.1999.0015
Quinn AA, Elkins KM (2017) The differentiation of menstrual from venous blood and other body fluids on various substrates using ATR FT-IR spectroscopy. J Forensic Sci 62:197–204. https://doi.org/10.1111/1556-4029.13250
Sikirzhytskaya A, Sikirzhytski V, Lednev IK (2014) Raman spectroscopy coupled with advanced statistics for differentiating menstrual and peripheral blood. J Biophotonics 7:59–67. https://doi.org/10.1002/jbio.201200191
Roeder AD, Haas C (2013) mRNA profiling using a minimum of five mRNA markers per body fluid and a novel scoring method for body fluid identification. Int J Legal Med 127:707–721. https://doi.org/10.1007/s00414-012-0794-3
Liang Q, Sun H, Wu X, Ou X, Gao G, Jin Y, Tong D (2018) Development of new mRNA markers for the identification of menstrual blood. Ann Clin Lab Sci 48:55–62
Richard MLL, Harper KA, Craig RL, Onorato AJ, Robertson JM, Donfack J (2012) Evaluation of mRNA marker specificity for the identification of five human body fluids by capillary electrophoresis. Forensic Sci Int Genet 6:452–460
Bauer M, Patzelt D (2002) Evaluation of mRNA markers for the identification of menstrual blood. J Forensic Sci 47:1278–1282. https://doi.org/10.1520/JFS15560J
Juusola J, Ballantyne J (2005) Multiplex mRNA profiling for the identification of body fluids. Forensic Sci Int 152:1–12. https://doi.org/10.1016/j.forsciint.2005.02.020
Wang Z, Zhang J, Luo H, Ye Y, Yan J, Hou Y (2013) Screening and confirmation of microRNA markers for forensic body fluid identification. Forensic Sci Int Genet 7:116–123. https://doi.org/10.1016/j.fsigen.2012.07.006
Silva SS (2015) Forensic miRNA : potential biomarker for body fluids ? Forensic Sci Int Genet 14:1–10. https://doi.org/10.1016/j.fsigen.2014.09.002
Orphanou C-M (2015) The detection and discrimination of human body fluids using ATR FT-IR spectroscopy. Forensic Sci Int 252:e10–e16. https://doi.org/10.1016/j.forsciint.2015.04.020
Jackson M, Mantsch HH (1995) The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit Rev Biochem Mol Biol 30:95–120. https://doi.org/10.3109/10409239509085140
Wood BR, Quinn MA, Tait B, Ashdown M, Hislop T, Romeo M, McNaughton D (1998) FTIR microspectroscopic study of cell types and potential confounding variables in screening for cervical malignancies. Biospectroscopy 4:75–91. https://doi.org/10.1002/(SICI)1520-6343(1998)4:2<75::AID-BSPY1>3.0.CO;2-R
Kazarian SG, Chan KLA (2006) Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim Biophys Acta Biomembr 1758:858–867. https://doi.org/10.1016/j.bbamem.2006.02.011
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta Bioenerg 1767:1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Ollesch J, Drees SL, Heise HM, Behrens T, Brüning T, Gerwert K (2013) FTIR spectroscopy of biofluids revisited: an automated approach to spectral biomarker identification. Analyst 138:4092–4102. https://doi.org/10.1039/C3AN00337J
Goodpaster JV, Liszewski EA (2009) Forensic analysis of dyed textile fibers. Anal Bioanal Chem 394:2009–2018. https://doi.org/10.1007/s00216-009-2885-7
Manheim J, Doty KC, McLaughlin G, Lednev IK (2016) Forensic hair differentiation using attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl Spectrosc 70:1109–1117. https://doi.org/10.1177/0003702816652321
Sonnex E, Almond MJ, Baum JV, Bond JW (2014) Identification of forged Bank of England £20 banknotes using IR spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 118:1158–1163. https://doi.org/10.1016/j.saa.2013.09.115
Harkins TR, Harris JT, Shreve OD (1959) Identification of pigments in paint products by infrared spectroscopy. Anal Chem 31:541–545. https://doi.org/10.1021/ac50164a025
Bueno J, Sikirzhytski V, Lednev IK (2013) ATR-FTIR spectroscopy for gunshot residue analysis: potential for ammunition determination. Anal Chem 85:7287–7294. https://doi.org/10.1021/ac4011843
Asri MNM, Nur Syuhaila Mat Desa W, Ismail D (2015) Fourier transform infrared (FTIR) spectroscopy with chemometric techniques for the classification of ballpoint pen inks. AJFSFM 1:194–200. https://doi.org/10.12816/0017699
Causin V, Casamassima R, Marega C, Maida P, Schiavone S, Marigo A, Villari A (2008) The discrimination potential of ultraviolet-visible spectrophotometry, thin layer chromatography, and Fourier transform infrared spectroscopy for the forensic analysis of black and blue ballpoint inks. J Forensic Sci 53:1468–1473. https://doi.org/10.1111/j.1556-4029.2008.00867.x
Lapachinske SF, Okai GG, dos Santos A, de Bairros AV, Yonamine M (2015) Analysis of cocaine and its adulterants in drugs for international trafficking seized by the Brazilian Federal Police. Forensic Sci Int 247:48–53. https://doi.org/10.1016/j.forsciint.2014.11.028
Yadav PK, Sharma RM (2019) Classification of illicit liquors based on their geographic origin using attenuated total reflectance (ATR) – Fourier transform infrared (FT-IR) spectroscopy and chemometrics. Forensic Sci Int 295:e1–e5. https://doi.org/10.1016/j.forsciint.2018.12.017
Sharma V, Bhardwaj S, Kumar R (2019) On the spectroscopic investigation of Kohl stains via ATR-FTIR and multivariate analysis: application in forensic trace evidence. Vib Spectrosc 101:81–91. https://doi.org/10.1016/j.vibspec.2019.02.006
Gładysz M, Król M, Kościelniak P (2017) Differentiation of red lipsticks using the attenuated total reflection technique supported by two chemometric methods. Forensic Sci Int 280:130–138. https://doi.org/10.1016/j.forsciint.2017.09.019
Sharma V, Bharti A, Kumar R (2019) On the spectroscopic investigation of lipstick stains: forensic trace evidence. Spectrochim Acta A Mol Biomol Spectrosc 215:48–57. https://doi.org/10.1016/j.saa.2019.02.093
De Wael K, Lepot L, Gason F, Gilbert B (2008) In search of blood—detection of minute particles using spectroscopic methods. Forensic Sci Int 180:37–42. https://doi.org/10.1016/j.forsciint.2008.06.013
Elkins KM (2011) Rapid presumptive “fingerprinting” of body fluids and materials by ATR FT-IR spectroscopy. J Forensic Sci 56:1580–1587. https://doi.org/10.1111/j.1556-4029.2011.01870.x
Gregório I, Zapata F, Torre M, García-Ruiz C (2017) Statistical approach for ATR-FTIR screening of semen in sexual evidence. Talanta 174:853–857. https://doi.org/10.1016/j.talanta.2017.07.016
Gregório I, Zapata F, García-Ruiz C (2017) Analysis of human bodily fluids on superabsorbent pads by ATR-FTIR. Talanta 162:634–640. https://doi.org/10.1016/j.talanta.2016.10.061
Lin H, Zhang Y, Wang Q, Li B, Huang P, Wang Z (2017) Estimation of the age of human bloodstains under the simulated indoor and outdoor crime scene conditions by ATR-FTIR spectroscopy. Sci Rep 7:13254. https://doi.org/10.1038/s41598-017-13725-1
Lin H, Zhang Y, Wang Q, Li B, Fan S, Wang Z (2018) Species identification of bloodstains by ATR-FTIR spectroscopy: the effects of bloodstain age and the deposition environment. Int J Legal Med 132:667–674. https://doi.org/10.1007/s00414-017-1634-2
Zhang Y, Wang Q, Li B, Wang Z, Li C, Yao Y, Huang P, Zhanyuan W (2017) Changes in attenuated total reflection Fourier transform infrared spectra as blood dries out. J Forensic Sci 62:761–767. https://doi.org/10.1111/1556-4029.13324
Takamura A, Watanabe K, Akutsu T, Ozawa T (2018) Soft and robust identification of body fluid using Fourier transform infrared spectroscopy and chemometric strategies for forensic analysis. Sci Rep 8:8459. https://doi.org/10.1038/s41598-018-26873-9
Takamura A, Watanabe K, Akutsu T, Ikegaya H, Ozawa T (2017) Spectral mining for discriminating blood origins in the presence of substrate interference via attenuated total reflection Fourier transform infrared spectroscopy: postmortem or antemortem blood? Anal Chem 89:9797–9804. https://doi.org/10.1021/acs.analchem.7b01756
Kumar R, Sharma V (2018) Chemometrics in forensic science. TrAC Trends Anal Chem 105:191–201. https://doi.org/10.1016/j.trac.2018.05.010
Gautam R, Vanga S, Ariese F, Umapathy S (2015) Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech Instrum 2:8. https://doi.org/10.1140/epjti/s40485-015-0018-6
Wu J (2014) Research on several problems in partial least squares regression analysis. Open Electr Electron Eng J 8:754–758
Adams KM (1979) Linear discriminant analysis in clinical neuropsychology research. J Clin Neuropsychol 1:259–272. https://doi.org/10.1080/01688637908414455
Morillas AV, Gooch J, Frascione N (2018) Feasibility of a handheld near infrared device for the qualitative analysis of bloodstains. Talanta 184:1–6. https://doi.org/10.1016/j.Talanta.2018.02.110
Unscrambler X (CAMO Software AS, Oslo, Norway)
Zapata F, de la Ossa MÁF, García-Ruiz C (2016) Differentiation of body fluid stains on fabrics using external reflection Fourier transform infrared spectroscopy (FT-IR) and chemometrics. Appl Spectrosc 70:654–665. https://doi.org/10.1177/0003702816631303
Acknowledgments
The authors would sincerely like to thank the University Grants Commission (UGC), Ministry of Human Resource Development, Govt. of India, for financial assistance and for providing laboratory facilities in the Department of Forensic Science, Punjabi University, Patiala.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the Institutional Ethical Committee (IEC), Punjabi University, Patiala, 147002, with letter number IEC/03-2017/08. All the participants were informed about the study and their consent was duly recorded.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, S., Chophi, R. & Singh, R. Forensic discrimination of menstrual blood and peripheral blood using attenuated total reflectance (ATR)-Fourier transform infrared (FT-IR) spectroscopy and chemometrics. Int J Legal Med 134, 63–77 (2020). https://doi.org/10.1007/s00414-019-02134-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-019-02134-w