Abstract
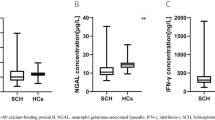
Previous studies reported that peripheral inflammation was associated with cognitive performance and brain structure in schizophrenia. However, the moderating effect of inflammation has not been extensively studied. This study investigated whether inflammation markers moderated the association between negative symptoms and neurocognition in schizophrenia. This cross-sectional study included 137 drug-naïve schizophrenia patients (DNS) and 67 healthy controls (HC). We performed the Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) for cognitive assessment and the Positive and Negative Syndrome Scale (PANSS) for psychiatric symptoms. Plasma concentrations of interferon-gamma (IFN-γ), neutrophil gelatinase-associated lipocalin (NGAL), and nuclear factor kappa B (NF-κB) were measured. The MCCB neurocognition score, social cognition score, and total score; the plasma concentrations of NGAL, IFN-γ, and NF-κB were significantly decreased in DNS than in HC (all P’s < 0.001). PANSS negative subscale (PNS), PANSS reduced expressive subdomain (RES) negatively correlated with neurocognition score (P = 0.007; P = 0.011, respectively). Plasma concentrations of IFN-γ and NGAL positively correlated with neurocognition score (P = 0.043; P = 0.008, relatively). The interactions of PNS × NGAL; PNS × IFN-γ; RES × IFN-γ accounted for significant neurocognition variance (P = 0.025; P = 0.029, P = 0.007, respectively). Simple slope analysis showed that all the above moderating effects only occurred in patients with near normal IFN-γ and NGAL levels. Plasma concentrations of IFN-γ and NGAL moderated the relationship between negative symptoms (especially RES) and neurocognition in schizophrenia. Treatment targeting inflammation may contribute to neurocognition improvement in schizophrenia.

Similar content being viewed by others
Data availability
The data that supported the findings of the study are available from the corresponding author upon reasonable request.
References
Elvevåg B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14(1):1–21
Sharma T, Antonova L (2003) Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am 26(1):25–40. https://doi.org/10.1016/s0193-953x(02)00084-9
García RR, Aliste F, Soto G (2018) Social cognition in schizophrenia: cognitive and neurobiological aspects. Rev Colomb Psiquiatr (Engl Ed) 47(3):170–176. https://doi.org/10.1016/j.rcp.2017.03.004
Sampedro A, Peña J, Ibarretxe-Bilbao N, Sánchez P, Iriarte-Yoller N, Pavón C, Hervella I, Tous-Espelosin M, Ojeda N (2020) Neurocognitive, social cognitive, and clinical predictors of creativity in schizophrenia. J Psychiatr Res 129:206–213. https://doi.org/10.1016/j.jpsychires.2020.06.019
Bang M, Kim KR, Song YY, Baek S, Lee E, An SK (2015) Neurocognitive impairments in individuals at ultra-high risk for psychosis: Who will really convert? Aust N Z J Psychiatry 49(5):462–470. https://doi.org/10.1177/0004867414561527
Bora E, Binnur Akdede B, Alptekin K (2017) Neurocognitive impairment in deficit and non-deficit schizophrenia: a meta-analysis. Psychol Med 47(14):2401–2413. https://doi.org/10.1017/s0033291717000952
Mohn C, Olsson AK, Johansson M, Moradi H, Helldin L (2021) Marginal relationship between affective dispositions and neurocognitive function in patients with schizophrenia spectrum disorders. Nord J Psychiatry 75(5):344–350. https://doi.org/10.1080/08039488.2020.1862294
Leifker FR, Bowie CR, Harvey PD (2009) Determinants of everyday outcomes in schizophrenia: the influences of cognitive impairment, functional capacity, and symptoms. Schizophr Res 115(1):82–87. https://doi.org/10.1016/j.schres.2009.09.004
Peuskens J, Demily C, Thibaut F (2005) Treatment of cognitive dysfunction in schizophrenia. Clin Ther 27(Suppl A):S25-37. https://doi.org/10.1016/j.clinthera.2005.07.015
Carbon M, Correll CU (2014) Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr 1(19 Suppl):38–52. https://doi.org/10.1017/s1092852914000601. (quiz 35-37, 53)
Zhu MH, Liu ZJ, Hu QY, Yang JY, Jin Y, Zhu N, Huang Y, Shi DH, Liu MJ, Tan HY, Zhao L, Lv QY, Yi ZH, Wu FC, Li ZZ (2022) Amisulpride augmentation therapy improves cognitive performance and psychopathology in clozapine-resistant treatment-refractory schizophrenia: a 12-week randomized, double-blind, placebo-controlled trial. Mil Med Res 9(1):59. https://doi.org/10.1186/s40779-022-00420-0
Chan RCK, Geng FL, Lui SSY, Wang Y, Ho KKY, Hung KSY, Gur RE, Gur RC, Cheung EFC (2015) Course of neurological soft signs in first-episode schizophrenia: relationship with negative symptoms and cognitive performances. Sci Rep 5:11053. https://doi.org/10.1038/srep11053
Chang WC, Tang JY, Hui CL, Wong GH, Chan SK, Lee EH, Chen EY (2013) The relationship of early premorbid adjustment with negative symptoms and cognitive functions in first-episode schizophrenia: a prospective three-year follow-up study. Psychiatry Res 209(3):353–360. https://doi.org/10.1016/j.psychres.2013.02.014
Rek-Owodziń K, Tyburski E, Plichta P, Waszczuk K, Bielecki M, Wietrzyński K, Podwalski P, Rudkowski K, Michalczyk A, Grąźlewski T, Sagan L, Kucharska-Mazur J, Samochowiec J, Mak M (2022) The relationship between cognitive functions and psychopathological symptoms in first episode psychosis and chronic schizophrenia. J Clin Med. https://doi.org/10.3390/jcm11092619
Brébion G, Bressan RA, Ohlsen RI, David AS (2013) A model of memory impairment in schizophrenia: cognitive and clinical factors associated with memory efficiency and memory errors. Schizophr Res 151(1–3):70–77. https://doi.org/10.1016/j.schres.2013.09.009
Hartmann-Riemer MN, Hager OM, Kirschner M, Bischof M, Kluge A, Seifritz E, Kaiser S (2015) The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr Res 169(1–3):427–432. https://doi.org/10.1016/j.schres.2015.10.032
Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H (2013) Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res 47(6):718–725. https://doi.org/10.1016/j.jpsychires.2013.01.024
Wu Q, Wang X, Wang Y, Long YJ, Zhao JP, Wu RR (2021) Developments in biological mechanisms and treatments for negative symptoms and cognitive dysfunction of schizophrenia. Neurosci Bull 37(11):1609–1624. https://doi.org/10.1007/s12264-021-00740-6
Lim J, Lee SA, Lam M, Rapisarda A, Kraus M, Keefe RS, Lee J (2016) The relationship between negative symptom subdomains and cognition. Psychol Med 46(10):2169–2177. https://doi.org/10.1017/s0033291716000726
Bora E (2019) Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med 49(12):1971–1979. https://doi.org/10.1017/s0033291719001685
Çakici N, Sutterland AL, Penninx B, Dalm VA, de Haan L, van Beveren NJM (2020) Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav Immun 88:547–558. https://doi.org/10.1016/j.bbi.2020.04.039
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21(12):1696–1709. https://doi.org/10.1038/mp.2016.3
Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, Walder K, Warren N (2023) Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry 10(4):260–271. https://doi.org/10.1016/s2215-0366(23)00025-1
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2(3):258–270. https://doi.org/10.1016/s2215-0366(14)00122-9
Na KS, Jung HY, Kim YK (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:277–286. https://doi.org/10.1016/j.pnpbp.2012.10.022
Campana M, Strauß J, Münz S, Oviedo-Salcedo T, Fernando P, Eichhorn P, Falkai P, Hasan A, Wagner E (2022) Cerebrospinal fluid pathologies in schizophrenia-spectrum disorder-a retrospective chart review. Schizophr Bull 48(1):47–55. https://doi.org/10.1093/schbul/sbab105
Jacomb I, Stanton C, Vasudevan R, Powell H, O’Donnell M, Lenroot R, Bruggemann J, Balzan R, Galletly C, Liu D, Weickert CS, Weickert TW (2018) C-reactive protein: higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Front Immunol 9:2230. https://doi.org/10.3389/fimmu.2018.02230
Ji E, Boerrigter D, Cai HQ, Lloyd D, Bruggemann J, O’Donnell M, Galletly C, Lloyd A, Liu D, Lenroot R, Weickert TW, Shannon Weickert C (2022) Peripheral complement is increased in schizophrenia and inversely related to cortical thickness. Brain Behav Immun 101:423–434. https://doi.org/10.1016/j.bbi.2021.11.014
North HF, Bruggemann J, Cropley V, Swaminathan V, Sundram S, Lenroot R, Pereira AM, Zalesky A, Bousman C, Pantelis C, Weickert TW, Shannon Weickert C (2021) Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur Arch Psychiatry Clin Neurosci 271(4):595–607. https://doi.org/10.1007/s00406-021-01237-z
Adamowicz DH, Shilling PD, Palmer BW, Nguyen TT, Wang E, Liu C, Tu X, Jeste DV, Irwin MR, Lee EE (2022) Associations between inflammatory marker profiles and neurocognitive functioning in people with schizophrenia and non-psychiatric comparison subjects. J Psychiatr Res 149:106–113. https://doi.org/10.1016/j.jpsychires.2022.02.029
Baek SH, Kim H, Kim JW, Ryu S, Lee JY, Kim JM, Shin IS, Kim SW (2022) Association between peripheral inflammatory cytokines and cognitive function in patients with first-episode schizophrenia. J Pers Med. https://doi.org/10.3390/jpm12071137
Chumakov E, Dorofeikova M, Tsyrenova K, Petrova N (2022) A cross-sectional study on associations between BDNF, CRP, IL-6 and clinical symptoms, cognitive and personal performance in patients with paranoid schizophrenia. Front Psychiatry 13:943869. https://doi.org/10.3389/fpsyt.2022.943869
Murphy CE, Walker AK, Weickert CS (2021) Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Transl Psychiatry 11(1):528. https://doi.org/10.1038/s41398-021-01607-0
Murphy CE, Walker AK, O’Donnell M, Galletly C, Lloyd AR, Liu D, Weickert CS, Weickert TW (2022) Peripheral NF-κB dysregulation in people with schizophrenia drives inflammation: putative anti-inflammatory functions of NF-κB kinases. Transl Psychiatry 12(1):21. https://doi.org/10.1038/s41398-021-01764-2
Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP (2009) The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiat 65(6):481–488. https://doi.org/10.1016/j.biopsych.2008.10.018
Wang M, Feng LR, Li ZL, Ma KG, Chang KW, Chen XL, Yang PB, Ji SF, Ma YB, Han H, Ruganzua JB, Yang WN, Qian YH (2021) Thymosin β4 reverses phenotypic polarization of glial cells and cognitive impairment via negative regulation of NF-κB signaling axis in APP/PS1 mice. J Neuroinflammation 18(1):146. https://doi.org/10.1186/s12974-021-02166-3
Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, Cao Y, Fisher M, Liu CZ (2020) Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-mediated TLR4/MyD88/NF-κB signaling pathway. Oxid Med Cell Longev 2020:8253904. https://doi.org/10.1155/2020/8253904
Chiang SS, Riedel M, Schwarz M, Mueller N (2013) Is T-helper type 2 shift schizophrenia-specific? Primary results from a comparison of related psychiatric disorders and healthy controls. Psychiatry Clin Neurosci 67(4):228–236. https://doi.org/10.1111/pcn.12040
Larsen JB, Reitan SK, Løberg EM, Rettenbacher M, Bruserud Ø, Larsen TK, Anda L, Bartz-Johannessen C, Johnsen E, Kroken RA (2021) The association between cytokines and psychomotor speed in a spectrum of psychotic disorders: a longitudinal study. Brain Behav Immun Health 18:100392. https://doi.org/10.1016/j.bbih.2021.100392
Wilson KE, Demyanovich H, Rubin LH, Wehring HJ, Kilday C, Kelly DL (2018) Relationship of interferon-γ to cognitive function in midlife women with schizophrenia. Psychiatr Q 89(4):937–946. https://doi.org/10.1007/s11126-018-9591-6
Yao C, Niu L, Fu Y, Zhu X, Yang J, Zhao P, Sun X, Ma Y, Li S, Li J (2021) Cognition, motor symptoms, and glycolipid metabolism in Parkinson’s disease with depressive symptoms. J Neural Transm (Vienna, Austria: 1996). https://doi.org/10.1007/s00702-021-02437-6
Lew LC, Hor YY, Yusoff NAA, Choi SB, Yusoff MSB, Roslan NS, Ahmad A, Mohammad JAM, Abdullah M, Zakaria N, Wahid N, Sun Z, Kwok LY, Zhang H, Liong MT (2019) Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clinical nutrition (Edinburgh, Scotland) 38(5):2053–2064. https://doi.org/10.1016/j.clnu.2018.09.010
He Z, Yang Y, Xing Z, Zuo Z, Wang R, Gu H, Qi F, Yao Z (2020) Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice. Cell Death Dis 11(6):440. https://doi.org/10.1038/s41419-020-2644-4
Naudé PJW, Ramakers I, van der Flier WM, Jiskoot LC, Reesink FE, Claassen J, Koek HL, Eisel ULM, De Deyn PP (2021) Serum and cerebrospinal fluid neutrophil gelatinase-associated lipocalin (NGAL) levels as biomarkers for the conversion from mild cognitive impairment to Alzheimer’s disease dementia. Neurobiol Aging 107:1–10. https://doi.org/10.1016/j.neurobiolaging.2021.07.001
Wei L, Du Y, Wu W, Fu X, Xia Q (2018) Elevation of plasma neutrophil gelatinase-associated lipocalin (NGAL) levels in schizophrenia patients. J Affect Disord 226:307–312. https://doi.org/10.1016/j.jad.2017.10.002
Wu CY, Bawa KK, Ouk M, Leung N, Yu D, Lanctôt KL, Herrmann N, Pakosh M, Swardfager W (2020) Neutrophil activation in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis of protein markers in blood and cerebrospinal fluid. Ageing Res Rev 62:101130. https://doi.org/10.1016/j.arr.2020.101130
Ferreira AC, Pinto V, Mesquita SD, Novais A, Sousa JC, Correia-Neves M, Sousa N, Palha JA, Marques F (2013) Lipocalin-2 is involved in emotional behaviors and cognitive function. Front Cell Neurosci 7:122. https://doi.org/10.3389/fncel.2013.00122
Gouweleeuw L, Hovens IB, van Leeuwen BL, Schoemaker RG (2017) Neutrophil gelatinase-associated lipocalin and microglial activity are associated with distinct postoperative behavioral changes in rats. Behav Brain Res 319:104–109. https://doi.org/10.1016/j.bbr.2016.11.023
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276. https://doi.org/10.1093/schbul/13.2.261
Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G (2014) Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiat 71(9):1058–1065. https://doi.org/10.1001/jamapsychiatry.2014.1105
Galderisi S, Mucci A, Buchanan RW, Arango C (2018) Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5(8):664–677. https://doi.org/10.1016/s2215-0366(18)30050-6
Bagney A, Dompablo M, Santabárbara J, Moreno-Ortega M, Lobo A, Jimenez-Arriero MA, Palomo T, Rodriguez-Jimenez R (2015) Are negative symptoms really related to cognition in schizophrenia? Psychiatry Res 230(2):377–382. https://doi.org/10.1016/j.psychres.2015.09.022
Dominguez Mde G, Viechtbauer W, Simons CJ, van Os J, Krabbendam L (2009) Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol Bull 135(1):157–171. https://doi.org/10.1037/a0014415
Hovington CL, Lepage M (2012) Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother 12(1):53–69. https://doi.org/10.1586/ern.11.173
İnce E, Üçok A (2018) Relationship between persistent negative symptoms and findings of neurocognition and neuroimaging in schizophrenia. Clin EEG Neurosci 49(1):27–35. https://doi.org/10.1177/1550059417746213
Leanza L, Egloff L, Studerus E, Andreou C, Heitz U, Ittig S, Beck K, Uttinger M, Riecher-Rössler A (2018) The relationship between negative symptoms and cognitive functioning in patients at clinical high risk for psychosis. Psychiatry Res 268:21–27. https://doi.org/10.1016/j.psychres.2018.06.047
Bittner RA, Linden DE, Roebroeck A, Härtling F, Rotarska-Jagiela A, Maurer K, Goebel R, Singer W, Haenschel C (2015) The when and where of working memory dysfunction in early-onset schizophrenia-a functional magnetic resonance imaging study. Cerebral cortex (New York, NY: 1991) 25(9):2494–2506. https://doi.org/10.1093/cercor/bhu050
Pu S, Nakagome K, Itakura M, Iwata M, Nagata I, Kaneko K (2016) The association between cognitive deficits and prefrontal hemodynamic responses during performance of working memory task in patients with schizophrenia. Schizophr Res 172(1–3):114–122. https://doi.org/10.1016/j.schres.2016.01.045
Na KS, Kim YK (2007) Monocytic, Th1 and th2 cytokine alterations in the pathophysiology of schizophrenia. Neuropsychobiology 56(2–3):55–63. https://doi.org/10.1159/000111535
Guo J, Liu C, Wang Y, Feng B, Zhang X (2015) Role of T helper lymphokines in the immune-inflammatory pathophysiology of schizophrenia: systematic review and meta-analysis. Nord J Psychiatry 69(5):364–372. https://doi.org/10.3109/08039488.2014.986761
Romeo B, Brunet-Lecomte M, Martelli C, Benyamina A (2018) Kinetics of cytokine levels during antipsychotic treatment in schizophrenia: a meta-analysis. Int J Neuropsychopharmacol 21(9):828–836. https://doi.org/10.1093/ijnp/pyy062
Eftekharian MM, Omrani MD, Arsang-Jang S, Taheri M, Ghafouri-Fard S (2019) Serum cytokine profile in schizophrenic patients. Hum Antibodies 27(1):23–29. https://doi.org/10.3233/hab-180344
Lesh TA, Careaga M, Rose DR, McAllister AK, Van de Water J, Carter CS, Ashwood P (2018) Cytokine alterations in first-episode schizophrenia and bipolar disorder: relationships to brain structure and symptoms. J Neuroinflamm 15(1):165. https://doi.org/10.1186/s12974-018-1197-2
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70(7):663–671. https://doi.org/10.1016/j.biopsych.2011.04.013
Demir I, Toker A, Zengin S, Laloglu E, Aksoy H (2016) Oxidative stress and insulin resistance in policemen working shifts. Int Arch Occup Environ Health 89(3):407–412. https://doi.org/10.1007/s00420-015-1079-1
Roudkenar MH, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M (2011) Neutrophil gelatinase-associated lipocalin: a new antioxidant that exerts its cytoprotective effect independent on Heme Oxygenase-1. Free Radical Res 45(7):810–819. https://doi.org/10.3109/10715762.2011.581279
Naudé PJ, Eisel UL, Comijs HC, Groenewold NA, De Deyn PP, Bosker FJ, Luiten PG, den Boer JA, Oude Voshaar RC (2013) Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J Psychosom Res 75(5):444–450. https://doi.org/10.1016/j.jpsychores.2013.08.023
de Bartolomeis A, Barone A, Vellucci L, Mazza B, Austin MC, Iasevoli F, Ciccarelli M (2022) Linking inflammation, aberrant glutamate-dopamine interaction, and post-synaptic changes: translational relevance for schizophrenia and antipsychotic treatment: a systematic review. Mol Neurobiol 59(10):6460–6501. https://doi.org/10.1007/s12035-022-02976-3
Misiak B, Stańczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D (2018) Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res 192:16–29. https://doi.org/10.1016/j.schres.2017.04.015
Çakici N, Sutterland AL, Penninx B, de Haan L, van Beveren NJM (2021) Changes in peripheral blood compounds following psychopharmacological treatment in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Psychol Med 51(4):538–549. https://doi.org/10.1017/s0033291721000155
Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S (2010) A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71(2):138–149. https://doi.org/10.4088/JCP.08m04666yel
Wu X, Shen Q, Chang H, Li J, Xing D (2022) Promoted CD4(+) T cell-derived IFN-γ/IL-10 by photobiomodulation therapy modulates neurogenesis to ameliorate cognitive deficits in APP/PS1 and 3xTg-AD mice. J Neuroinflammation 19(1):253. https://doi.org/10.1186/s12974-022-02617-5
Borregaard N, Sørensen OE, Theilgaard-Mönch K (2007) Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28(8):340–345. https://doi.org/10.1016/j.it.2007.06.002
Dekens DW, Eisel ULM, Gouweleeuw L, Schoemaker RG, De Deyn PP, Naudé PJW (2021) Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res Rev 70:101414. https://doi.org/10.1016/j.arr.2021.101414
Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ (2009) Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology 34(8):1885–1903. https://doi.org/10.1038/npp.2009.26
Zhao J, He X, Liu Z, Yang D (2006) The effects of clozapine on cognitive function and regional cerebral blood flow in the negative symptom profile schizophrenia. Int J Psychiatry Med 36(2):171–181. https://doi.org/10.2190/1aa0-uw9q-1cnk-3e2n
Javitt DC (1999) Treatment of negative and cognitive symptoms. Curr Psychiatry Rep 1(1):25–30. https://doi.org/10.1007/s11920-999-0007-z
McGurk SR (1999) The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry 60(Suppl 12):24–29
Park KH, Park ES, Jo SM, Seo MH, Song YO, Jang SJ (2021) Effects of a short emotional management program on inpatients with schizophrenia: a pilot study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18105497
Ruiz-Iriondo M, Salaberria K, Polo-López R, Iruín A, Echeburúa E (2019) Preventing cognitive decline in chronic schizophrenia: long-term effectiveness of integrated psychological therapy and emotional management training. Psicothema 31(2):114–120. https://doi.org/10.7334/psicothema2018.254
Ruiz-Iriondo M, Salaberría K, Polo-López R, Iruin Á, Echeburúa E (2020) Improving clinical symptoms, functioning, and quality of life in chronic schizophrenia with an integrated psychological therapy (IPT) plus emotional management training (EMT): a controlled clinical trial. Psychother Res 30(8):1026–1038. https://doi.org/10.1080/10503307.2019.1683634
Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, Kuwahara Y, Fukumoto M, Shokrgozar MA (2008) Neutrophil gelatinase-associated lipocalin acts as a protective factor against H(2)O(2) toxicity. Arch Med Res 39(6):560–566. https://doi.org/10.1016/j.arcmed.2008.05.003
Martinuzzi E, Barbosa S, Daoudlarian D, Bel Haj Ali W, Gilet C, Fillatre L, Khalfallah O, Troudet R, Jamain S, Fond G, Sommer I, Leucht S, Dazzan P, McGuire P, Arango C, Diaz-Caneja CM, Fleischhacker W, Rujescu D, Glenthøj B, Winter I, Kahn RS, Yolken R, Lewis S, Drake R, Davidovic L, Leboyer M, Glaichenhaus N (2019) Stratification and prediction of remission in first-episode psychosis patients: the OPTiMiSE cohort study. Transl Psychiatry 9(1):20. https://doi.org/10.1038/s41398-018-0366-5
Nettis MA, Pergola G, Kolliakou A, O’Connor J, Bonaccorso S, David A, Gaughran F, Di Forti M, Murray RM, Marques TR, Blasi G, Bertolino A, Pariante CM, Dazzan P, Mondelli V (2019) Metabolic-inflammatory status as predictor of clinical outcome at 1-year follow-up in patients with first episode psychosis. Psychoneuroendocrinology 99:145–153. https://doi.org/10.1016/j.psyneuen.2018.09.005
Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC (2019) An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med 49(14):2307–2319. https://doi.org/10.1017/s0033291719001995
Fard MT, Cribb L, Nolidin K, Savage K, Wesnes K, Stough C (2020) Is there a relationship between low-grade systemic inflammation and cognition in healthy people aged 60–75 years? Behav Brain Res 383:112502. https://doi.org/10.1016/j.bbr.2020.112502
Acknowledgements
We thank all of the study participants for their cooperation.
Funding
This work was supported in part by the Natural Science Foundation of Tianjin Municipal Science and Technology Bureau (22JCZDJC00110); Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-033A); Tianjin Key Medical Discipline (Specialty) Construction Project of Tianjin Health Commission (TJWJ2022XK039); Foundation of Tianjin Health Commission for Young Scholars (KJ20067).
Author information
Authors and Affiliations
Contributions
JL: designed the study and wrote the study protocol. ML, YQ, GL, SY, WS, XZ, and YZ: acquired the data. XS and YL: completed the ELISA. ML: performed the statistical analyses. JL, ML, and YQ: interpreted the data and drafted the initial manuscript. All authors contributed to the final drafting and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors report no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Luo, G., Qiu, Y. et al. Negative symptoms and neurocognition in drug-naïve schizophrenia: moderating role of plasma neutrophil gelatinase-associated lipocalin (NGAL) and interferon-gamma (INF-γ). Eur Arch Psychiatry Clin Neurosci (2023). https://doi.org/10.1007/s00406-023-01650-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-023-01650-6