Abstract
Evidence for the effectiveness of physical activity (PA) in the treatment of depression prevails for outpatients with mild and moderate symptom levels. For inpatient treatment of severe depression, evidence-based effectiveness exists only for structured and supervised group PA interventions. The Step Away from Depression (SAD) study investigated the effectiveness of an individual pedometer intervention (PI) combined with an activity diary added to inpatient treatment as usual (TAU). In this multicenter randomized controlled trial, 192 patients were randomized to TAU or TAU plus PI. The two primary outcomes at discharge were depression—blindly rated with the Montgomery–Åsberg Depression Rating Scale (MADRS)—and average number of daily steps measured by accelerometers. Secondary outcomes were self-rated depression and PA, anxiety, remission and response rates. Multivariate analysis of variance (MANOVA) revealed no significant difference between both groups for depression and daily steps. Mean MADRS scores at baseline were 29.5 (SD = 8.3) for PI + TAU and 28.8 (SD = 8.1) for TAU and 16.4 (SD = 10.3) and 17.2 (SD = 9.9) at discharge, respectively. Daily steps rose from 6285 (SD = 2321) for PI + TAU and 6182 (SD = 2290) for TAU to 7248 (SD = 2939) and 7325 (SD = 3357). No differences emerged between groups in secondary outcomes. For severely depressed inpatients, a PI without supervision or further psychological interventions is not effective. Monitoring, social reinforcement and motivational strategies should be incorporated in PA interventions for this population to reach effectiveness.
Similar content being viewed by others
Introduction
Depression has a tremendous burden on people’s health [1]. It is one of the most lethal mental diseases with a 1.51 to 1.90 higher death risk (Hazard’s Ratio) for patients compared to healthy persons [2,3,4]. Furthermore, severe additional health risks like stroke or cardiovascular diseases are associated with depression [5, 6]. Research shows several causes for these risks—a generally less healthy life style, with worse nutrition and less physical activity, as well as (neuro-) biological and neuroendocrinological processes [7,8,9,10,11,12].
Treatment costs for depression are the highest compared to all other mental diseases [13, 14]. Psychotherapy and antidepressant medication represent the main treatment options [15, 16]. More severe depressive disorders go along with more expensive treatments—inpatient treatment accounts for almost half of the direct treatment costs [17]. The search for both—higher clinical effectiveness and lower costs of the treatment of depression—is an urgent task in mental health care. Our trial is meant to contribute to that matter.
Physical activity already plays an important role in the prevention of depression [18,19,20,21,22]. Even more strongly, as part of antidepressant treatment, it is increasingly recommended during last years [15, 16, 23]. The effectiveness of physical activity in the treatment of depression was shown in many reviews and confirmed in several meta-analyses [24,25,26,27,28,29]. Besides, results of no effectiveness have been equally reported [30], leaving questions about contradicting findings. A number of interventions have been developed and implemented [31, 32]. However, most studies and interventions focus on mild to moderate depression symptom level and on outpatient treatment [29, 33]. Little work has been done for patients with severe depression that undergo inpatient treatment. Thus, we are interested in new findings concerning PA interventions for this relevant population.
Furthermore, we want to include the aspect of integrated health care because it helps lowering costs and makes treatment more efficient [34,35,36]. Therefore, we look for interventions that offer the chance of being continued on the long term after inpatient treatment in an outpatient setting [37] and that can be supported by non-specialized health care personnel. An easy and inexpensive method for increasing PA is the use of pedometers combined with the formulation of step goals [38]. Pedometers can be easily used by patients and provide the potential of cross-sectional use for both inpatient and outpatient treatment. While 10.000 steps per day were originally propagated by a Japanese company to promote commercial pedometers, research shows that already 7000 steps have beneficial effects on health [39]. Daily step counts lower than 4000 are associated with higher mortality [40].”
To our knowledge, no study so far investigated the add-on-effect of a pedometer intervention for inpatients with depression. In this trial, we examine the effectiveness of a pedometer intervention combined with the use of an activity book as an add-on-intervention for the inpatient treatment of severe depression.
Methods
Trial design and overview
This multicenter, longitudinal, randomized controlled parallel-group trial was conducted in ten psychiatric clinics in Germany and Austria: Charité Universitätsmedizin Berlin, Alexianer St. Hedwig Hospital, University Hospital RWTH Aachen, DIAKO Hospital Flensburg, Hospital LMU Munich, University Hospital Frankfurt, University Hospital Göttingen, University Hospital Salzburg, Oberhavel Clinic Hennigsdorf and Health Center Odenwaldkreis. The study was registered as a clinical trial (see Supplementary Materials S1, ClinicalTrials.gov Identifier: NCT02850341, registered January 08, 2016). A detailed study protocol was published containing the description of methodological and statistical aspects of the study [41]. Treatment as usual (TAU) plus a pedometer intervention (PI) was compared to TAU only at baseline (t0) and at discharge (t1b). If t1b data were missing, we replaced it by t1a data (4 weeks after hospital admission) as last observation carried forward. Points of measurement altogether were day 1–3 after hospital admission (baseline, t0), after 4 weeks (t1a) (if treatment length extended 4 weeks), at discharge (t1b), and 6 months after hospital admission (follow-up). Enrollment took place from August 2016 until January 2020.
Study population and randomization
Patients were included with an age between 18 and 65 years and with major depression as primary diagnosis. Psychiatrists in each clinic in charge of the patient performed the clinical diagnosis by taking the medical history, using additional questionnaires and preexisting clinical health records of the patient. In addition, a planned treatment length of 4 weeks was needed. Exclusion diagnoses were psychotic depression, borderline personality disorder, schizophrenia, anorexia nervosa, current substance addiction and dementia. Further reasons for exclusion were medical risks or inability to walk at least 5000 steps, current use of a pedometer or other activity tracker, as well as a baseline level of more than 10.000 steps per day. Patients were randomized in a 1:1 ratio stratified by center using a computerized random number generator. Randomization remained concealed until the end of the baseline measurement.
Study procedure
Investigators in all centers were clinical staff and/or advanced students of psychology or medicine, all trained in standard conduction of the trial and of the assessments. Patients underwent baseline measurements within the first three working days of hospital treatment. They filled out questionnaires online via SoSci-Survey [42] or, in the occurrence of any technical or personal constraints, by paper–pencil. At baseline only, we collected sociodemographic variables, number of former depressive episodes and treatments, somatic diseases, medication and physiological parameters (blood pressure, heart rate, body weight, blood glucose, and laboratory values of triglycerides, cholesterol, high-density lipoprotein and low density lipoprotein). All other measures were collected at every measure point (see Supplementary Materials S2). TAU consisted of inpatient psychotherapy (group and/or individual), pharmacotherapy, adjuvant sociotherapy, occupational therapy, music therapy, physiotherapy and/or exercise therapy. Patients of the TAU plus PI received an Omron Walking style IV pedometer (Model HJ-325-EW) and an activity diary. Originating from the patient’s initially blinded number of steps as baseline measurement, they were instructed to raise their daily number of steps by 500 for the coming week. If the goal of 500 additional daily steps was fulfilled on at least four days of one week and 10.000 steps had not been reached yet, the goal for step count should be raised again by 500 daily steps for the next week. Patients had to fill in their daily steps into the diary throughout the whole hospital treatment and continuing afterward for altogether 6 months maximum.
Outcome measures
Our two primary outcomes were the MADRS sum score and average daily step count at discharge. Trained raters blind to the group assignment of the patient conducted the MADRS interview. Trainer’s interrater correlations showed high reliability with Cronbach’s Alpha of 0.978 (CI: 0.95–0.99) [41]. Step count was assessed with ActiGraph GT1M accelerometers (ActiGraph) [43,44,45]. Patients wore the accelerometers around their waist for three consecutive days during wake time. The outcome variable was mean daily step count (valid if at least two days with at least 10 h of wear time).
As secondary outcomes we used the International Physical Activity Questionnaire (IPAQ) [46], the Beck Depression Inventory II (BDI-II) [47] and the Beck Anxiety Inventory (BAI) [48]. Exploratory, we collected nine further measures for post hoc analyses: (1) psychopathological symptoms, (2) health-related quality of life, (3) self-reported depressive symptoms, for physical activity: (4) self-efficacy, (5) intention, (6) self-concordance of goal-striving, (7) outcome expectations, (8) planning and barrier planning and (9) general self-efficacy. In addition, we collected data concerning the adherence to and the evaluation of the intervention, treatment history, critical life events during the follow-up time interval and adverse events during the trial (see Supplementary Materials S2).
Sample size and power calculation
Based on other add-on exercise studies [30, 49,50,51], we assumed a small to moderate effect size for both differences in MADRS sum score and mean step count. Results of these trials showed an absolute mean difference of 4 points in the MADRS [50]. A difference of 1.6–1.9 in the MADRS score represents a minimal clinically meaningful change [52]. A difference of 4 points on the MADRS is associated with reduction of 0.5 in the CGI [53]. We therefore estimated a mean difference of four points in the MADRS score as a substantially meaningful clinical difference between treatment groups. Pedometer studies showed an increase of approximately 1000 steps per day in the intervention group [51, 54]. 1000 steps more per day are associated with essential improvements for individual health [39]. Thus, we expected a number of 1000 steps as a mean difference between groups. For reaching a power of 95% in a parallel group, fixed sample trial with multivariate analysis of variance (MANOVA), 264 cases were necessary according to G*Power Version 3.2.1). Taking into account a loss rate of 34% for dropout and missing data, we aimed at an overall sample size of 400 patients.
Statistical analysis
In our primary analysis, we analyzed patients with complete data. In our second full analysis set, data of all eligible and randomized patients were analyzed. For this set, missing data of the two outcome variables MADRS and mean daily steps were imputed with multiple imputations (number of imputations: 10; relative efficiency: 94–96%). The multiple imputation model was based on age, sex, hospitalization length, body weight and the following variables, respectively, for baseline, t1a, t1b and follow-up: step count, MADRS, BDI-II, time in light, moderate, vigorous, very vigorous PA (tresholds of Freedson [55], using ActiLife Software [56]), sedentary time, IPAQ and BAI. All data were analyzed using SPSS Statistics 25. Significance levels were set to p = 0.05.
First, we calculated descriptive statistics for the sociodemographic and clinical data of patients. Remission and response rates were calculated, as well as percentages of patients meeting the WHO recommendations for PA [57].
As specified in our study protocol, the two primary outcomes depression and steps were likely to correlate [58] and we planned a multivariate analysis of variance (MANOVA) for them as dependent variables with group as independent variable. However, our results showed no significant correlation between depression and steps. Furthermore, both dependent variables were not normally distributed (showing significant results in the Kolmogorov-Smirnoff-Test). Homogeneity was given (Box’s M = 9.96, p = 0.18). According to Finch et al. [59], under these conditions MANOVA is still more powerful than nonparametric tests so we continued the analysis with MANOVA. Secondary outcomes of groups were compared using ANOVA. Differences in frequencies between groups were calculated using exact Fisher’s test (if only two variables were compared) or Pearson’s Chi-square test. We used Bonferroni correction to adjust for multiple testing.
Results
Participant characteristics and missing data
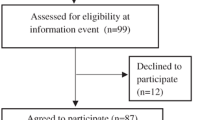
Altogether, 732 eligible patients were asked to participate. 315 of them consented, leading to an acceptance rate of 43.03 percent. However, enrollment took place from July 2016 to January 2020, for limits in staff and resources impeded an further extension of the trial. Figure 1 shows the flow diagram with additional information about non-participants and dropouts. Our final sample size to analyze was N = 192 (N = 83 TAU plus PI, N = 109 TAU), providing a power of 0.86 for a parallel group, fixed sample trial with multivariate analysis of variance (MANOVA) with p = 0.05 (using G*Power Version 3.1.9.4).
Of 732 eligible persons asked, 315 consented to participate, Patients excluded or refusing to participate after baseline measurement had an age, sex, educational level, MADRS score or daily step count that was comparable to those participating (see Supplementary Materials S3a and S3b).
Sixty (72%) of the TAU + PI and 82 (75%) of the TAU participants with complete data could be used in the primary analysis. Trial sites showed significantly different rates of cases with complete data, ranging from 41.67 to 100.00%, χ2 = 21.4, df = 9, p = 0.01 (with the constraint of a 30% cell frequency < 5).
22% of the participants in the TAU + PI and 18% in the TAU group dropped out of the study until discharge. 14 (7.2%) patients gave no reasons for their quitting of the trial, 12 (6.2%) did lose their interest in the study. 10 (5.2%) were discharged earlier than 4 weeks after baseline. One patient (0.5%) was injured and one patient (0.5%) felt overtaxed by the trial. Dropout patients had a significant lower BDI-II score at baseline (mean [SD] BDI-II score, 26.3 [12.3]) than completers (mean [SD] BDI-II score, 32.0 [10.6]; t = 2.86, df = 183, p = 0.005). No significant differences emerged for trial site, treatment condition, age, sex, educational level, steps per day, MADRS and IPAQ.
Participants completing the full study protocol had missing data of 6.02% of the TAU + PI and 8.26% of the TAU group for the primary outcomes. For the full analysis set with all 192 cases, we included complete cases and imputed cases of dropout and missing data.
Baseline group characteristics are shown in Table 1. Groups were comparable regarding most variables including age, sex and the primary outcomes. Overall, patients were 41.7 years old (SD = 13.3), 106 (55%) were women, 98 (52%) had a vocational and 47 (25%) an academic educational level. 74 patients (39%) were seeking work and 84 (44%) were (self-)employed. Mean MADRS score at baseline was 29 (SD = 8.3) and mean steps per day were 6222 (SD = 2300). Mean treatment length was 49 days (Median = 43; SD = 27) with no difference between groups.
Differences in sociodemographic, clinical and outcome variables between the different trial centers were marginal and are presented in detail in Supplementary Materials S4.
Primary outcomes
Mean MADRS score at discharge was 16.4 (SD = 10.3) in the TAU + PI and 17.2 (SD = 9.9) in the TAU group. Mean steps per day were 7248 (SD = 2939) and 7325 (SD = 3357), respectively (see Table 2).
The correlation between the two primary outcomes was not significant (rs(85) = − 0.12, p = 0.25). Figure 2 presents the comparison of the two treatment groups concerning means and standard deviations for the primary outcomes at baseline and discharge.
Our MANOVA in the primary analysis II set showed no significant difference between groups for both dependent variables (F (2, 84) = 0.67, p = 0.52 (Pillai’s trace V = 0.16)). Likewise, in the imputed primary analysis I set, MANOVA in all of the 10 data sets of the multiple imputation showed no significant difference between treatments (see Supplementary Materials S5).
No differences emerged in response and remission rates. Forty-six percent of the TAU + PI group and 40% in the TAU group had more than 50% reduction in the MADRS from baseline to discharge (see Supplementary Materials S6). Remission rate was 25% (14/57) for the TAU + PI and 18% (14/72) for the TAU group.
The percentage of participants in each group meeting criteria of WHO-recommended PA at discharge was higher when self-rated. Measured with accelerometers, 44% of the TAU + PI and 43% of the TAU group met WHO recommendations. For the self-rated IPAQ, these were 58% and 51%, respectively.
Secondary outcomes
Groups showed no significant differences in means for both treatment groups at discharge for IPAQ, BDI-II and BAI. In the IPAQ, total weekly physical activity (estimated by weighting time spent in each activity intensity with its estimated metabolic equivalent task (MET) energy expenditure) of the TAU + PI group was 4200.87 MET minutes per week (SD = 4539.00). The TAU group showed 4213.89 MET minutes per week (SD = 5891.25). The means of the BDI-II were 19.39 (SD = 11.86) and 20.94 (SD = 13.29), those of the BAI 15.79 (SD = 11.63) and 15.19 (SD = 11.24), respectively. Further results of secondary outcomes are presented in Supplementary Materials S7.
Additional exploratory analyses
Likewise, no outcome differences emerged between groups in exploratory analyses for subgroups of patients with different sex, younger or older patients or patients with moderate versus severe depression according to MADRS cut-off value of 35 points. However, for severely depressed patients only, the correlation between steps per day and depression at baseline was significant, r(25) = − 0.61, p < 0.001 (see Supplementary Materials S8e). For moderately depressed patients, this correlation remained non-significant. Furthermore, treatment effect was not different between responders and non-responders.
Besides, we explored the amount of PA on different weekdays at baseline. Patients showed least steps on Sundays (5375.92, SD = 2617.95) and Mondays (5678.71, SD = 2892.30) and most steps on Tuesdays (7269.32, SD = 2389.30) and Saturdays (6800.33, SD = 3204.88). Detailed information on all exploratory results can be found in Supplementary Materials S8.
Adverse events, compliance and acceptance of the intervention
No serious adverse events were reported throughout the study course.
We received copies of the activity book of 56 participants (93% of TAU + PI participants of T1 measurements). Compliance with the PI decreased in the course of treatment. Figure 3 shows compliance rates over all 24 weeks starting with 98.2% of patients using the activity book in the first, 91.1% in the fourth, 57.1% in the 8th and 25% in the 28th week. Those participants who kept the diary did so with diminishing completeness: Whereas in the first week, patients filled out 95.1% of the activity book, this percentage declined to 93.4% in the second, 87.8% in the third and 80.9% in the fourth week.
Full information about results of the program evaluation can be found in Supplementary Materials S9 and S10. The PI was rated as moderately helpful by participants with a mean score of 4.1 (SD = 1.3, N = 69) on a 1 (“not helpful at all”) to 6 (“very helpful”) Likert scale. Of 69 participants answering the evaluation questionnaire, 42 (61%) said that they were more physically active because of the PI. Using grades of 1 (very good) to 6 (insufficient) participants evaluated the PI with 2.1 (SD = 0.9). Main openly formulated positive evaluations of the PI were that it was “motivating” (13/35, 37%), that “monitoring helped” (8/35, 23%) and that it gave more control about the amount of physical activity performed (4/35, 11%). As disadvantages participants mentioned the “accelerometer measurement” (5/35, 14%), “nothing” (4/35, 11%) and that “steps were too biased as a measure for PA” (3/35, 9%). As possible improvements “the use of a fitness wristband” (3/35, 9%) and “more support” (2/35, 6%) were suggested.
Discussion
This multicenter trial, to our knowledge, was the first to investigate the effect of an add-on pedometer intervention in the inpatient treatment of depression. Results showed no significant differences between treatment groups. Neither depressive symptoms, nor step level were different between patients with additional PI and patients undergoing TAU only. Equally, we found no differences in secondary outcome measures between groups. This was unexpected, for research has shown that PA has a significant effect in the treatment of depression [24, 25].
One reason for our results could be the comparatively high step levels of our sample at baseline. This contradicts decreased PA of depressive patients generally observed in other studies [60, 61]. However, this is in line with findings that inpatients move more than outpatients [62] and that pedometer (or accelerometer) wearing leads to an increase in PA [38]. These high baseline values could have diminished the chance to measure between-group-differences. For further research projects in naturalistic settings, routine measurement of PA for every patient should be integrated into inpatient treatment so that this effect can be reduced as much as possible. Moreover, using an armwrist tool may attract less attention and lead to a more reliable measurement of PA [63]. All the more, continuous measuring with armwrist tools can provide the possibility of measuring heart rate, heart rate variability and rest and sleep times.
A second reason could be that compliance and adherence rates of patients decreased over weeks. Thus, our intervention may not have been successfully implemented in the course of treatment. If adherence is ensured by participation of inpatients in structured physical exercise like aerobic sessions, studies show that PA as an add-on to TAU significantly helps decreasing depression [64, 65]. We do not know how effective the PI would be when thoroughly adhered to by patients. Adherence problems were reported already in other PA trials with depressive patients [66, 67]. Therefore, our results are in line with other researchers suggesting that adherence to PA interventions should be supported by behavior change interventions [28, 68,69,70,71]. For instance, developing concrete action plans, barrier management and working with situational cues might be useful adherence-increasing strategies to be implemented in PA interventions. Furthermore, positive affective reactions while performing PA need to be supported—PA interventions should meet these requirements, e.g., by paying attention to positive environment and fun rather than pressure. Finally, regular monitoring and reward for the patients if keeping up with PA should be included.
Third, also patients of the TAU condition could have tried to make more steps during treatment because they were motivated by the mere presence of the conducted trial in the psychiatric ward. It could not be hidden that the aim of the PI was to increase PA and this was known to TAU patients. Additionally, it cannot be ruled out that our study attracted patients more strongly motivated to increase their PA than those not participating which could have biased results.
Our dropout rates were as expected and comparable to other exercise (as well as psychotherapy) studies for depression [72, 73]. Therefore, we can assume that the study design as well as the conduct of the trial were of good quality and did not bias results.
Interestingly, the correlation between depression and steps was not statistically significant. This was contrary to our expectations as other studies showed significant correlations between depression and PA [58]. It is possible that in our specific population of depressive inpatients the general relationship between these two variables is different from that for outpatients. Notably, another recent study showed a significant negative correlation between depression and PA for outpatients but not for inpatients [74]. In our subgroup analysis, we found a significant negative correlation only for patients with severe depression. Thus, it might be that the correlation between PA and depression might only be significant for patients with either light or severe depression but may be less distinct for patients with moderate depression. Further research is needed to assess this question.
Moreover, the measurement of steps could have constraints with regard to its reliability. Admission as well as discharge weeks of treatment might be different from usual PA of depressive patients. Moreover, we recorded steps for three consecutive days. Also, we did not differentiate between days in the week and on the weekend. Results show that patients move less on Sundays and Mondays. This might be a specific finding for inpatient treatment. Sundays have already been shown to be associated with less PA, in general. Reduction in PA on Mondays in psychiatric wards could be specifically due to more waiting time for visits of the attending doctors or to more routine examinations. Hence, these differences of PA for different weekdays should be taken into account for further research in an inpatient population.
On the one hand, maybe a period of 5 or 7 days would have led to other estimates of daily step level. On the other hand, adherence rates would then possibly be reduced due to a higher burden. However, three days of monitoring were shown to be sufficient for reliable and valid estimates of PA, especially, if the sample is large enough [75,76,77].
Alternative explanations for our results may also be that exercise is not causally connected with decrease in depression [30], that personality traits may be a non-considered variable [78] or that the role of genes needs to be regarded, too [79, 80]. In addition, individual profit as well as contraindication might have happened that cannot be shown by focusing on means of data but would tribute individualized statistical methods.
In our study, the sample fulfilled the WHO recommendations for PA as much as patients with severe mental diseases [62] and—as expected—lower compared to healthy samples [81]. Rates of self-rated measures were lower. This confirms the discrepancy between subjective and objective measurement of PA and the need to integrate both types of measurement in exercise research [41]. Some studies showed weak validity of the IPAQ [82] and using other self-report questionnaires for PA with higher psychometric quality such as the simple physical activity questionnaire (SIMPAQ) [83] may be of advantage.
To sum up, although participants subjectively evaluated the intervention as helpful, our results implicate that, in inpatient treatment, the use of a pedometer and an activity book alone does not lead to an additional significant change of depression or step level on the long term. This is in line with studies showing that patients need more support to maintain motivation and to develop volitional strategies [84, 85]. Regular supervision of the program would be promising [23, 68] and patients of our study made this wish (see Supplementary Materials S10). Additionally, emotional reactions [86] and smartphone use as a tool option [87] should be considered.
Limitations and strengths
We examined a simple non-expensive PA intervention with an excellent balance of potential benefits and risks in a naturalistic setting of inpatient treatment for depressive patients in a multicenter design. We measured depression as well as PA with two measures, respectively, including blinded clinical rating of depression severity accelerometry-based PA, and we provided long-term data during and follow-up data after the release from hospital.
Restrained power was a limitation of our trial due to recruitment of only 315 instead of planned 400 subjects as well as substantial loss of data. Even having used multiple imputation for the analysis I, power still provided a 14% chance of not detecting a true effect. However, power was sufficiently strong to detect a small effect in primary outcomes. For the analysis II with complete data cases, results would have been underpowered with 0.74. Therefore, we cannot totally rule out the possibility of a type II error not detecting an effect.
Furthermore, contribution of several trial sites to the final study sample was unequal. However, we found no differences in baseline values or results when analyzing data center-wise. Double-blindness was not possible and acceptance rate was low. This could have contributed to a bias in results. Finally, the high baseline PA of patients was unexpected. Selection of participants might have been biased in the way that patients with no interest in PA could have preferred not to participate in the study. This could have reduced the chance of finding an effect of our intervention for depressive inpatients with low PA. Future studies should pay attention to attract especially patients with low PA for the inclusion of PA trials.
Conclusion
We found that adding a PI to TAU without further supervision or psychological interventions does not lead to less depression severity or more steps at release from hospital of depressive inpatients. This can be due to high effectiveness of inpatient treatment in general and low adherence for the PI. As a conclusion, adherence to PA interventions should be supported by regular supervision. Future research work in this field should plan, describe and implement adherence-supporting strategies when using PA interventions for patients with depression.
Data availability
The data of this study and analyses presented in the paper are available upon request from first author.
References
World Health Organization (2017) Depression and other common mental disorders: Global health estimates World Health Organization, Geneva
Cohen BE, Edmondson D, Kronish IM (2015) State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 28(11):1295–1302. https://doi.org/10.1093/ajh/hpv047
Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I (2017) Depression and mortality in a longitudinal study: 1952–2011. CMAJ 189(42):E1304–E1310. https://doi.org/10.1503/cmaj.170125
Walker ER, McGee RE, Druss BG (2015) Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiat 72(4):334–341. https://doi.org/10.1001/jamapsychiatry.2014.2502
Nicholson A, Kuper H, Hemingway H (2006) Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 27(23):2763–2774. https://doi.org/10.1093/eurheartj/ehl338
Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB (2011) Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA 306(11):1241–1249. https://doi.org/10.1001/jama.2011.1282
Bautista LE, Vera-Cala LM, Colombo C, Smith P (2012) Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens 25(4):505–511. https://doi.org/10.1038/ajh.2011.256
Kronish IM, Rieckmann N, Halm EA, Shimbo D, Vorchheimer D, Haas DC et al (2006) Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 21(11):1178–1183. https://doi.org/10.1111/j.1525-1497.2006.00586.x
Slavich GM, Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140(3):774–815. https://doi.org/10.1037/a0035302
Grippo AJ, Johnson AK (2009) Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12(1):1–21. https://doi.org/10.1080/10253890802046281
Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG et al (2017) Dietary patterns and depression risk: a meta-analysis. Psychiatry Res 253:373–382. https://doi.org/10.1016/j.psychres.2017.04.020
Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M (2018) Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord 226:346–354. https://doi.org/10.1016/j.jad.2017.09.022
Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A et al (2016) US spending on personal health care and public health, 1996–2013. JAMA 316(24):2627–2646. https://doi.org/10.1001/jama.2016.16885
Thomas CM, Morris S (2003) Cost of depression among adults in England in 2000. Br J Psychiatry 183(6):514–519. https://doi.org/10.1192/00-000
DGPPN B KBV, AWMF, AkdÄ, BPtK, BApK, DAGSHG, DEGAM, DGPM, DGPs, DGRW (Editors) for the Guideline Group Unipolar Depression (2015) S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression - Langfassung, 2. Auflage AWMF für die Leitliniengruppe Unipolare Depression,
American Psychiatric Association (2019) clinical practice guideline for the treatment of depression across three age cohorts. Am Psychiatric Pub 173(5):543–546
Krauth C, Stahmeyer JT, Petersen JJ, Freytag A, Gerlach FM, Gensichen J (2014) Resource utilisation and costs of depressive patients in Germany: results from the primary care monitoring for depressive patients trial. Depress Res Treat 2014:730891. https://doi.org/10.1155/2014/730891
Fernandez-Montero A, Moreno-Galarraga L, Sanchez-Villegas A, Lahortiga-Ramos F, Ruiz-Canela M, Martinez-Gonzalez MA et al (2020) Dimensions of leisure-time physical activity and risk of depression in the “Seguimiento Universidad de Navarra” (SUN) prospective cohort. BMC Psychiatry 20(1):98. https://doi.org/10.1186/s12888-020-02502-6
Gianfredi V, Blandi L, Cacitti S, Minelli M, Signorelli C, Amerio A et al (2020) Depression and objectively measured physical activity: a systematic review and meta-analysis. Int J Environ Res Public Health 17(10):3738. https://doi.org/10.3390/ijerph17103738
Mammen G, Faulkner G (2013) Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med 45(5):649–657. https://doi.org/10.1016/j.amepre.2013.08.001
Stroehle A, Hofler M, Pfister H, Muller AG, Hoyer J, Wittchen HU et al (2007) Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med 37(11):1657–1666. https://doi.org/10.1017/S003329170700089X
Silva LRB, Seguro CS, de Oliveira CGA, Santos POS, de Oliveira JCM, de Souza Filho LFM et al (2020) Physical inactivity is associated with increased levels of anxiety, depression, and stress in Brazilians during the COVID-19 pandemic: a cross-sectional study. Front Psychiatry 11:565291. https://doi.org/10.3389/fpsyt.2020.565291
Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M et al (2018) EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 54:124–144. https://doi.org/10.1016/j.eurpsy.2018.07.004
Miller KJ, Gonçalves-Bradley DC, Areerob P, Hennessy D, Mesagno C, Grace F (2020) Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials. Ageing Res Rev 58:100999. https://doi.org/10.1016/j.arr.2019.100999
Mutrie N, Richards K, Lawrie S, Mead G (2018) Can physical activity prevent or treat clinical depression? In: Budde H, Wegner M (eds) The exercise effect on mental health Routledge. London p, New York, pp 380–407
Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B (2016) Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res 77:42–51. https://doi.org/10.1016/j.jpsychires.2016.02.023
Stroehle A (2009) Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) 116(6):777–784. https://doi.org/10.1007/s00702-008-0092-x
Xie Y, Wu Z, Sun L, Zhou L, Wang G, Xiao L et al (2021) The Effects and Mechanisms of Exercise on the Treatment of Depression. Front Psychiatry 12:705559. https://doi.org/10.3389/fpsyt.2021.705559
Imboden C, Claussen MC, Seifritz E, Gerber M (2021) Physical activity for the treatment and prevention of depression: a rapid review of meta-analyses. Deutsche Zeitschrif Sportmed 72(6):280–287. https://doi.org/10.5960/dzsm.2021.499
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR et al (2013) Exercise for depression. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004366.pub6
Busch AM, Ciccolo JT, Puspitasari AJ, Nosrat S, Whitworth JW, Stults-Kolehmainen M (2016) Preferences for exercise as a treatment for depression. Ment Health Phys Act 10:68–72. https://doi.org/10.1016/j.mhpa.2015.12.004
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES et al (2018) Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 175(7):631–648. https://doi.org/10.1176/appi.ajp.2018.17111194
Cooney G, Dwan K, Mead G (2014) Exercise for depression. JAMA 311(23):2432–2433. https://doi.org/10.1001/jama.2014.4930
Thota AB, Sipe TA, Byard GJ, Zometa CS, Hahn RA, McKnight-Eily LR et al (2012) Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med 42(5):525–538. https://doi.org/10.1016/j.amepre.2012.01.019
Williams JW Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A (2007) Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry 29(2):91–116. https://doi.org/10.1016/j.genhosppsych.2006.12.003
Butler M, Kane RL, McAlpine D, Kathol R, Fu SS, Hagedorn H et al (2011) Does integrated care improve treatment for depression? a systematic review. J Ambul Care Manage 34(2):113–125. https://doi.org/10.1097/JAC.0b013e31820ef605
Falkai P, Schmitt A, Rosenbeiger CP, Maurus IA-O, Hattenkofer L, Hasan A et al (2022) Aerobic exercise in severe mental illness: requirements from the perspective of sports medicine. Eur Arch Psychiatry Clin Neurosci 272:643–677. https://doi.org/10.1007/s00406-021-01360-x
Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R et al (2007) Using pedometers to increase physical activity and improve health: a systematic review. JAMA 298(19):2296–2304. https://doi.org/10.1001/jama.298.19.2296
Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B et al (2011) How many steps/day are enough? for adults. Int J Behav Nutr Phys Act 8(1):79. https://doi.org/10.1186/1479-5868-8-79
Saint-Maurice PF, Troiano RP, Bassett DR Jr, Graubard BI, Carlson SA, Shiroma EJ et al (2020) Association of daily step count and step intensity with mortality among US adults. JAMA 323(12):1151–1160. https://doi.org/10.1001/jama.2020.1382
Grosse J, Petzold MB, Brand R, Strohle A (2021) Step Away from Depression-Study protocol for a multicenter randomized clinical trial for a pedometer intervention during and after in-patient treatment of depression. Int J Methods Psychiatr Res 30(1):e1862. https://doi.org/10.1002/mpr.1862
Leiner D. SoSci Survey. Version 3.1.06. 2019.
Kaminsky LA, Ozemek C (2012) A comparison of the Actigraph GT1M and GT3X accelerometers under standardized and free-living conditions. Physiol Meas 33(11):1869–1876. https://doi.org/10.1088/0967-3334/33/11/1869
Petzold MB, Bischoff S, Rogoll J, Plag J, Teran C, Brand R et al (2017) Physical activity in outpatients with mental disorders: status, measurement and social cognitive determinants of health behavior change. Eur Arch Psychiatry Clin Neurosci 267(7):639–650. https://doi.org/10.1007/s00406-017-0772-3
Silva P, Mota J, Esliger D, Welk G (2010) Technical reliability assessment of the actigraph GT1M accelerometer. Meas Phys Educ Exerc Sci 14:79–91. https://doi.org/10.1080/10913671003715524
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395. https://doi.org/10.1249/01.mss.0000078924.61453.fb
Beck AT, Steer RA, Brown GK (1996) BDI-II, Beck depression inventory : manual The Psychological Corporation, San Antonio
Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56(6):893–897. https://doi.org/10.1037/0022-006X.56.6.893
Heinzel S, Lawrence JB, Kallies G, Rapp MA, Heissel A (2015) Using exercise to fight depression in older adults. GeroPsych 28(4):149–162. https://doi.org/10.1024/1662-9647/a000133
Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Rocha NS, Fleck MP (2015) Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res 61:25–32. https://doi.org/10.1016/j.jpsychires.2014.11.005
Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M et al (2011) A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care 49(7):641–648. https://doi.org/10.1097/MLR.0b013e318215d0c9
Duru G, Fantino B (2008) The clinical relevance of changes in the Montgomery-Asberg depression rating scale using the minimum clinically important difference approach. Curr Med Res Opin 24(5):1329–1335. https://doi.org/10.1185/030079908X291958
Leucht S, Fennema H, Engel RR, Kaspers-Janssen M, Lepping P, Szegedi A (2017) What does the MADRS mean? equipercentile linking with the CGI using a company database of mirtazapine studies. J Affect Disord 210:287–293. https://doi.org/10.1016/j.jad.2016.12.041
Abedi P, Nikkhah P, Najar S (2015) Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric 18(6):841–845. https://doi.org/10.3109/13697137.2015.1065246
Freedson PS, Melanson E, Sirard J (1998) Calibration of the Computer Science and Applications Inc. accelerometer. Med Sci Sports Exerc 30(5):777–781. https://doi.org/10.1097/00005768-199805000-00021
Wyatt J. Acitlife 6 User's Manual. 2012.
World Health Organization (2010) Global Recommendations on Physical Activity for Health WHO, Geneva 2010
Vancampfort D, Stubbs B, Sienaert P, Wyckaert S, De Hert M, Rosenbaum S et al (2015) What are the factors that influence physical activity participation in individuals with depression? a review of physical activity correlates from 59 studies. Psychiatr Danub 27(3):210–224
Finch H (2005) Comparison of the performance of nonparametric and parametric MANOVA test statistics when assumptions are violated. Methodology 1(1):27–38. https://doi.org/10.1027/1614-1881.1.1.27
de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BW (2010) Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress Anxiety 27(11):1057–1065. https://doi.org/10.1002/da.20738
Schuch F, Vancampfort D, Firth J, Rosenbaum S, Ward P, Reichert T et al (2017) Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord 210:139–150. https://doi.org/10.1016/j.jad.2016.10.050
Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M et al (2017) Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 16(3):308–315. https://doi.org/10.1002/wps.20458
O’Brien JT, Gallagher P, Stow D, Hammerla N, Ploetz T, Firbank M et al (2017) A study of wrist-worn activity measurement as a potential real-world biomarker for late-life depression. Psychol Med 47(1):93–102. https://doi.org/10.1017/S0033291716002166
Philippot A, Dubois V, Lambrechts K, Grogna D, Robert A, Jonckheer U et al (2022) Impact of physical exercise on depression and anxiety in adolescent inpatients: a randomized controlled trial. J Affect Disord 301:145–153. https://doi.org/10.1016/j.jad.2022.01.011
Imboden C, Gerber M, Beck J, Holsboer-Trachsler E, Puhse U, Hatzinger M (2020) Aerobic exercise or stretching as add-on to inpatient treatment of depression: similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. J Affect Disord 276:866–876. https://doi.org/10.1016/j.jad.2020.07.052
Kruisdijk F, Hopman-Rock M, Beekman ATF, Hendriksen I (2019) EFFORT-D: results of a randomised controlled trial testing the EFFect of running therapy on depression. BMC Psychiatry 19(1):170. https://doi.org/10.1186/s12888-019-2156-x
Krogh J, Videbech P, Thomsen C, Gluud C, Nordentoft M (2012) DEMO-II trial Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS ONE 7(10):e48316. https://doi.org/10.1371/journal.pone.0048316
Machaczek KK, Allmark P, Pollard N, Goyder E, Shea M, Horspool M et al (2021) Integrating physical activity into the treatment of depression in adults: a qualitative enquiry. Health Soc Care Community. https://doi.org/10.1111/hsc.13283
Schuch FB, Stubbs B (2019) The role of exercise in preventing and treating depression. Curr Sports Med Rep 18(8):299–304. https://doi.org/10.1249/JSR.0000000000000620
Glowacki K, Duncan MJ, Gainforth H, Faulkner G (2017) Barriers and facilitators to physical activity and exercise among adults with depression: A scoping review. Ment Health Phys Act 13:108–119. https://doi.org/10.1016/j.mhpa.2017.10.001
Petzold MB, Mumm JLM, Bischoff S, Grosse J, Plag J, Brand R et al (2019) Increasing physical activity and healthy diet in outpatients with mental disorders: a randomized-controlled evaluation of two psychological interventions. Eur Arch Psychiatry Clin Neurosci 269(5):529–542. https://doi.org/10.1007/s00406-018-0941-z
Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A et al (2016) Dropout from exercise randomized controlled trials among people with depression: a meta-analysis and meta regression. J Affect Disord 190:457–466. https://doi.org/10.1016/j.jad.2015.10.019
Cooper AA, Conklin LR (2015) Dropout from individual psychotherapy for major depression: a meta-analysis of randomized clinical trials. Clin Psychol Rev 40:57–65. https://doi.org/10.1016/j.cpr.2015.05.001
Görgülü EA-O, Bieber M, Engeroff T, Zabel K, Etyemez S, Prvulovic D et al (2021) Physical activity, physical self-perception and depression symptoms in patients with major depressive disorder: a mediation analysis. Eur Arch Psychiatry Clin Neurosci 271(1433–8491):1205–1215. https://doi.org/10.1007/s00406-021-01299-z
Wolff-Hughes DL, McClain JJ, Dodd KW, Berrigan D, Troiano RP (2016) Number of accelerometer monitoring days needed for stable group-level estimates of activity. Physiol Meas 37(9):1447–1455. https://doi.org/10.1088/0967-3334/37/9/1447
Bergman P, Hagstromer M (2020) No one accelerometer-based physical activity data collection protocol can fit all research questions. BMC Med Res Methodol 20(1):141. https://doi.org/10.1186/s12874-020-01026-7
Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L (2017) Measuring physical activity with hip accelerometry among us older adults: how many days are enough? PLoS ONE 12(1):e0170082. https://doi.org/10.1371/journal.pone.0170082
De Moor MHM, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJC (2008) Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry 65(8):897–905. https://doi.org/10.1001/archpsyc.65.8.897
de Geus EJC (2021) A genetic perspective on the association between exercise and mental health in the era of genome-wide association studies. Mental Health Phys Activity 20:100378. https://doi.org/10.1016/j.mhpa.2020.100378
Johnson W, Mortensen EL, Kyvik KO (2020) Gene-environment interplay between physical exercise and fitness and depression symptomatology. Behav Genet 50(5):346–362. https://doi.org/10.1007/s10519-020-10009-9
Guthold R, Stevens GA, Riley LM, Bull FC (2018) Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 6(10):e1077–e1086. https://doi.org/10.1016/s2214-109x(18)30357-7
Lee PH, Macfarlane DJ, Lam TH, Stewart SM (2011) Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 8:115. https://doi.org/10.1186/1479-5868-8-115
Rosenbaum S, Morell R, Abdel-Baki A, Ahmadpanah M, Anilkumar TV, Baie L et al (2020) Assessing physical activity in people with mental illness: 23-country reliability and validity of the simple physical activity questionnaire (SIMPAQ). BMC Psychiatry 20(1):108. https://doi.org/10.1186/s12888-020-2473-0
Zhang CQ, Zhang R, Schwarzer R, Hagger MS (2019) A meta-analysis of the health action process approach. Health Psychol 38(7):623–637. https://doi.org/10.1037/hea0000728
McCartan CJ, Yap J, Firth J, Stubbs B, Tully MA, Best P et al (2020) Factors that influence participation in physical activity for anxiety or depression: a synthesis of qualitative evidence. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.Cd013547
Brand R, Cheval B (2019) Theories to explain exercise motivation and physical inactivity: ways of expanding our current theoretical perspective. Front Psychol 10:1147. https://doi.org/10.3389/fpsyg.2019.01147
Romeo A, Edney S, Plotnikoff R, Curtis R, Ryan J, Sanders I et al (2019) Can smartphone apps increase physical activity? systematic review and meta-analysis. J Med Internet Res 21(3):e12053. https://doi.org/10.2196/12053
Acknowledgments
Authors like to thank the Robert-Enke-Stiftung and the “Referat Sportpsychiatrie und –psychotherapie” of the “Deutsche Gesellschaft für Psychiatrie und Psychotherapie, Psychosomatik und Nervenheilkunde e.V.” for supporting this trial. We are very grateful to all participants of this study as well as to all research workers, psychiatrists and nurses helping conducting the trial at all sites.
Funding
Open Access funding enabled and organized by Projekt DEAL. This trial was supported by the “Robert-Enke-Stiftung” with no role in planning, conducting, analysis or publication of the study (no grant number given). Open Access was funded by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to, read and approved this report of results.
Corresponding author
Ethics declarations
Conflict of interest
AS has received research support from Robert-Enke-Stiftung for this specific trial. JG has received a salary from that funding of the Robert-Enke-Stiftung. All other authors declare no conflicts of interest.
Ethical approval
The study was approved by the Ethics Commission of Charité Universitätsmedizin Berlin, Germany (EA1/088/16). All participants gave their written informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Große, J., Huppertz, C., Röh, A. et al. Step away from depression—results from a multicenter randomized clinical trial with a pedometer intervention during and after inpatient treatment of depression. Eur Arch Psychiatry Clin Neurosci 274, 709–721 (2024). https://doi.org/10.1007/s00406-023-01646-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01646-2