Abstract
Purpose
To investigate the clinical significance of programmed cell death ligand 1 (PD-L1) expression in ovarian clear cell carcinoma (CCC).
Materials and methods
Patients with CCC who underwent primary surgery at our hospital between 1984 and 2014 were enrolled in this study. PD-L1 and mismatch repair (MMR) protein expression in tumor cells, tumor-infiltrating lymphocytes (TILs), including cluster of differentiation (CD) 8, CD4, forkhead box P3 (FOXP3), programmed cell death 1 (PD-1), and BAF250a, were evaluated using immunohistochemistry. The association between PD-L1 expression, clinicopathological features, prognosis, and expression of several proteins was investigated.
Results
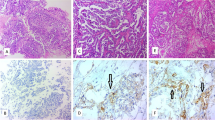
Of the 125 patients with CCC, 17 had negative PD-L1 and 108 had positive PD-L1. Patients with positive PD-L1 expression showed a lower response to chemotherapy (p = 0.01). In addition, patients with positive PD-L1 showed worse progression-free survival (PFS, p = 0.01) and overall survival (OS, p = 0.01) than that in patients with negative PD-L1 expression. Multivariate analyses for PFS and OS showed that PD-L1 expression was an independent prognostic factor for PFS (hazard ratio [HR] 7.81, p < 0.01) and OS (HR 12.90, p < 0.01). PD-L1 expression was not associated with the expression of several TILs or proteins.
Conclusion
The expression of PD-L1 was related to a lower response to chemotherapy and worse prognosis in CCC. These results may be useful for the development of new treatments.




Similar content being viewed by others
Availability of data and material
The corresponding author will send dataset according to reasonable request.
Code availability
Not applicable.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108. https://doi.org/10.3322/caac.21262
Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone G, Giurgea L, Timmers P, Coens C, Pecorelli S (2003) Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant Chemo Therapy in Ovarian neoplasm trial. J Natl Cancer Inst 95:113–125
Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20:1248–1259. https://doi.org/10.1200/JCO.2002.20.5.1248
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, Moriya T, Sugiyama T (2006) Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer 94:1369–1374. https://doi.org/10.1038/sj.bjc.6603116
Ryu SY, Park SI, Nam BH, Kim I, Yoo CW, Nam JH, Lee KH, Cho CH, Kim JH, Park SY, Kim BG, Kang SB (2009) Prognostic significance of histological grade in clear-cell carcinoma of the ovary: a retrospective study of Korean Gynecologic Oncology Group. Ann Oncol 20:1032–1036. https://doi.org/10.1093/annonc/mdn764
Fujiwara K, Shintani D, Nishikawa T (2016) Clear-cell carcinoma of the ovary. Ann Oncol 27(Supplement 1):i50–i52. https://doi.org/10.1093/annonc/mdw086
Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, Furuya K (2013) Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol 24:37–43. https://doi.org/10.3802/jgo.2013.24.1.37
Meng X, Huang Z, Teng F, Xing L, Yu J (2015) Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 41:868–876. https://doi.org/10.1016/j.ctrv.2015.11.001
Reiss KA, Forde PM, Brahmer JR (2014) Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy 6:459–475. https://doi.org/10.2217/imt.14.9
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y (2010) Tumor cell expression of programmed cell death-1 ligand1 is a prognostic factor for malignant melanoma. Cancer 116:1757–1766. https://doi.org/10.1002/cncr.24899
Chen YB, Mu CY, Huang JA (2012) Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 98:751–755. https://doi.org/10.1700/1217.13499
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH (2013) B7–H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 8:e76012. https://doi.org/10.1371/journal.pone.0076012
Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154:1805–1813. https://doi.org/10.1016/S0002-9440(10)65436-3
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20:5064–5074. https://doi.org/10.1158/1078-0432.CCR-13-3271
Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, Nagel ZD, Zou J, Wang C, Kapoor P, Ma X, Ma D, Liang J, Song S, Liu J, Samson LD, Ajani JA, Li GM, Liang H, Shen X, Mills GB, Peng G (2018) ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 24:556–562. https://doi.org/10.1038/s41591-018-0012-z
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 104:3360–3365. https://doi.org/10.1073/pnas.0611533104
Zhu J, Wen H, Bi R, Wu Y, Wu X (2017) Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol 28:e77. https://doi.org/10.3802/jgo.2017.28.e77
Xiao X, Dong D, He W, Song L, Wang Q, Yue J, Xie L (2018) Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol 149:146–154. https://doi.org/10.1016/j.ygyno.2018.02.009
Chui MH, Ryan P, Radigan J, Ferguson SE, Pollett A, Aronson M, Semotiuk K, Holter S, Sy K, Kwon JS, Soma A, Singh N, Gallinger S, Shaw P, Arseneau J, Foulkes WD, Gilks CB, Clarke BA (2014) The histomorphology of Lynch syndrome-associated ovarian carcinomas: toward a subtype-specific screening strategy. Am J Surg Pathol 38:1173–1181. https://doi.org/10.1097/PAS.0000000000000298
Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, D’Andrea AD, Matulonis UA, Konstantinopoulos PA (2017) Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology 6:e1277308. https://doi.org/10.1080/2162402X.2016.1277308
Bennett JA, Morales-Oyarvide V, Campbell S, Longacre TA, Oliva E (2016) Mismatch repair protein expression in clear cell carcinoma of the ovary: incidence and morphologic associations in 109 cases. Am J Surg Pathol 40:656–663. https://doi.org/10.1097/PAS.0000000000000602
Mutch DG, Prat J (2014) 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 133:401–404. https://doi.org/10.1016/j.ygyno.2014.04.013
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline, 1.1 version. Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48:434–452. https://doi.org/10.1016/j.immuni.2018.03.014
Oda K, Hamanishi J, Matsuo K, Hasegawa K (2018) Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol 151:381–389. https://doi.org/10.1016/j.ygyno.2018.09.001
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ, IeM S (2009) Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol 174:1597–1601. https://doi.org/10.2353/ajpath.2009.081000
Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Tsuta K, Nokihara H, Tamura T, Asamura H, Kawaishi M, Kuwano K, Ochiya T (2015) The clinical relevance of the miR-197/CKS1B/STAT3 -mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther 23:717–727. https://doi.org/10.1038/mt.2015.10
Wang H, Fu C, Du J, Wang H, He R, Yin X, Li H, Li X, Wang H, Li K, Zheng L, Liu Z, Qiu Y (2020) Enhanced histone H3 acetylation of the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for PD-L1 expression in drug-resistant cancer cells. J Exp Clin Cancer Res 39:29. https://doi.org/10.1186/s13046-020-1536-x
Ishibashi M, Tamura H, Sunakawa M, Kondo-Onodera A, Okuyama N, Hamada Y, Moriya K, Choi I, Tamada K, Inokuchi K (2016) Myeloma drug resistance induced by binding of myeloma B7–H1 (PD-L1) to PD-1. Cancer Immunol Res 4:779–788. https://doi.org/10.1158/2326-6066.CIR-15-0296
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SE, Schnitt SS, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O’Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 24:311–335. https://doi.org/10.1097/PAP.0000000000000161
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213. https://doi.org/10.1056/NEJMoa020177
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 102:18538–18543. https://doi.org/10.1073/pnas.0509182102
Stumpf M, Hasenburg A, Riener MO, Jütting U, Wang C, Shen Y, Orlowska-Volk M, Fisch P, Wang Z, Gitsch G, Werner M, Lassmann S (2009) Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br J Cancer 101:1513–1521. https://doi.org/10.1038/sj.bjc.6605274
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I (2015) Safety and antitumor activity of anti-PD-1 antibody, Nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015–4022. https://doi.org/10.1200/JCO.2015.62.3397
Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, Hamilton EP, Annunziata CM, Grote HJ, von Heydebreck A, Grewal J, Chand V, Gulley JL (2019) Efficacy and safety of Avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol 5:393–401. https://doi.org/10.1001/jamaoncol.2018.6258
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was conducted without any financial support.
Author information
Authors and Affiliations
Contributions
Protocol/project development: HM, MM, HT, and MT. Data collection or management: TH, HI, HI, SK, RS, TI, and JS. Data analysis: HM, MM, HT. Manuscript writing/editing: HM, MM, and MT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The present study was approved by the Ethics Committee of the National Defense Medical College Hospital (No. 2769).
Informed consent
Informed consent did not be obtained from all participants due to retrospective analysis.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuura, H., Miyamoto, M., Hada, T. et al. The worsening impact of programmed cell death ligand 1 in ovarian clear cell carcinomas. Arch Gynecol Obstet 306, 2133–2142 (2022). https://doi.org/10.1007/s00404-022-06582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06582-5