Abstract
Background
Molecular profiling of breast cancer (BC) classifies several intrinsic subtypes based on different patterns of gene expression. Multigene assays estimate the risk of recurrence and help to select high-risk patients for adjuvant chemotherapy. However, these tests are associated with significant costs. Immunohistochemistry (IHC) offers a surrogate classification for molecular subtypes by determining estrogen (ER) and progesterone receptors (PR), human epidermal growth factor (Her2neu), as well as the proliferation marker Ki67. Core needle biopsy (CNB) is well established in BC diagnosis and allows a pre-operative assessment of biomarkers. The aim of this study was to analyze the concordance of these markers between CNB and surgical specimens to assess whether re-testing of the surgical specimen is mandatory.
Materials and methods
Within a 3-year period, patients with primary BC and paired samples of CNB and surgical specimens were analyzed retrospectively. Concordance rates of ER, PR, Her2neu, Ki67, and the surrogate classification for molecular subtypes were calculated using the Landis and Koch agreement grades.
Results
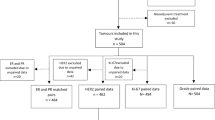
Out of 2254 patients with primary breast cancer, 1307 paired specimens without pre-operative treatment were available for analysis Concordance rates for ER, PR, Her2neu, and Ki67 status showed substantial-to-almost perfect agreement grades (κ = 0.91, 0.75, 0.89, and 0.61, respectively). Though substantial concordance was also found for the subtype classification (κ = 0.70), the molecular subtype changed in 18.5% of patients based on the testing of the surgical specimen, mainly from luminal A-like to luminal B-like.
Conclusions
Though the concordance rates for single markers were convincing, a significant proportion of the molecular subtypes differed between CNB and the surgical specimen. Re-testing of PR and Ki67 is mandatory to ensure optimal treatment decisions. Further research is necessary to define safe, efficient, and cost-effective predictive models in adjuvant breast cancer therapy.


Similar content being viewed by others
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. https://doi.org/10.1038/nature11412
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278. https://doi.org/10.1200/jco.2010.31.2835
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750. https://doi.org/10.1093/jnci/djp082
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thurlimann B, Andre F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Khaled H, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B (2018) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 29(10):2153. https://doi.org/10.1093/annonc/mdx806
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel m (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. https://doi.org/10.1056/NEJMoa041588
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Leitlinienprogramm (2020) Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Mammakarzinom_4_0/Version_4.3/LL_Mammakarzinom_Langversion_4.3.pdf
Chen J, Wang Z, Lv Q, Du Z, Tan Q, Zhang D, Xiong B, Zeng H, Gou J (2017) Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: a report of 1003 cases. Pathol Oncol Res 23(4):769–775. https://doi.org/10.1007/s12253-017-0187-5
Chen X, Sun L, Mao Y, Zhu S, Wu J, Huang O, Li Y, Chen W, Wang J, Yuan Y, Fei X, Jin X, Shen K (2013) Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC cancer 13:390. https://doi.org/10.1186/1471-2407-13-390
Chen X, Yuan Y, Gu Z, Shen K (2012) Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat 134(3):957–967. https://doi.org/10.1007/s10549-012-1990-z
Li S, Yang X, Zhang Y, Fan L, Zhang F, Chen L, Zhou Y, Chen X, Jiang J (2012) Assessment accuracy of core needle biopsy for hormone receptors in breast cancer: a meta-analysis. Breast Cancer Res Treat 135(2):325–334. https://doi.org/10.1007/s10549-012-2063-z
Robertson S, Ronnlund C, de Boniface J, Hartman J (2019) Re-testing of predictive biomarkers on surgical breast cancer specimens is clinically relevant. Breast Cancer Res Treat 174(3):795–805. https://doi.org/10.1007/s10549-018-05119-2
You K, Park S, Ryu JM, Kim I, Lee SK, Yu J, Kim SW, Nam SJ, Lee JE (2017) Comparison of core needle biopsy and surgical specimens in determining intrinsic biological subtypes of breast cancer with immunohistochemistry. J Breast Cancer 20(3):297–303. https://doi.org/10.4048/jbc.2017.20.3.297
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF, International Ki-67 in Breast Cancer Working G (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664. https://doi.org/10.1093/jnci/djr393
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, Gown AM, Symmans WF, Piper T, Mehl E, Enos RA, Hayes DF, Dowsett M, Nielsen TO (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906. https://doi.org/10.1093/jnci/djt306
Cserni G, Voros A, Liepniece-Karele I, Bianchi S, Vezzosi V, Grabau D, Sapino A, Castellano I, Regitnig P, Foschini MP, Zolota V, Varga Z, Figueiredo P, Decker T, Focke C, Kulka J, Kaya H, Reiner-Concin A, Amendoeira I, Callagy G, Caffrey E, Wesseling J, Wells C (2014) Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast 23(3):259–263. https://doi.org/10.1016/j.breast.2014.02.003
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F (2015) Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 24(Suppl 2):S67-72. https://doi.org/10.1016/j.breast.2015.07.017
Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97(17):1254–1261. https://doi.org/10.1093/jnci/dji249
Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98(9):1802–1810. https://doi.org/10.1002/cncr.11745
Berghuis AMS, van Deurzen CHM, Koffijberg H, Terstappen L, Sleijfer S, MJ IJ, (2019) Real-world data on discordance between estrogen, progesterone, and HER2 receptor expression on diagnostic tumor biopsy versus tumor resection material. Breast Cancer Res Treat 175(2):451–458. https://doi.org/10.1007/s10549-019-05141-y
Greer LT, Rosman M, Mylander WC, Hooke J, Kovatich A, Sawyer K, Buras RR, Shriver CD, Tafra L (2013) Does breast tumor heterogeneity necessitate further immunohistochemical staining on surgical specimens? J Am Coll Surg 216(2):239–251. https://doi.org/10.1016/j.jamcollsurg.2012.09.007
Tamaki K, Sasano H, Ishida T, Miyashita M, Takeda M, Amari M, Tamaki N, Ohuchi N (2010) Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci 101(9):2074–2079. https://doi.org/10.1111/j.1349-7006.2010.01630.x
Lundgren C, Bendahl PO, Borg A, Ehinger A, Hegardt C, Larsson C, Loman N, Malmberg M, Olofsson H, Saal LH, Sjoblom T, Lindman H, Klintman M, Hakkinen J, Vallon-Christersson J, Ferno M, Ryden L, Ekholm M (2019) Agreement between molecular subtyping and surrogate subtype classification: a contemporary population-based study of ER-positive/HER2-negative primary breast cancer. Breast Cancer Res Treat 178(2):459–467. https://doi.org/10.1007/s10549-019-05378-7
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MT, AK, NS, MH, and MP. The first draft of the manuscript was written by MP and OS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest concerning this paper. MP reports personal fees for serving on advisory boards and lecture honoraria from AstraZeneca, Teva, Clovis, and Roche. MB reports grants and personal fees from AstraZeneca, Celgene, Genomic Health, Medac, MSD, Novartis, Pfizer, Roche, Teva. MT, MH, NS, AK, C Brambs, C Becker, and OS are reporting no general conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pölcher, M., Braun, M., Tischitz, M. et al. Concordance of the molecular subtype classification between core needle biopsy and surgical specimen in primary breast cancer. Arch Gynecol Obstet 304, 783–790 (2021). https://doi.org/10.1007/s00404-021-05996-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-05996-x