Abstract
Purpose
Our purpose is to investigate the reasons why Lactobacillus iners is detected in abnormal vaginal microbial flora.
Methods
In this study, in vitro characteristics of four type strains (L. crispatus, L. iners, L. gasseri, and L. jensenii) were examined by measuring the growth speed by OD660, and acid resistance, with gram stain and Live/Dead stain.
Results
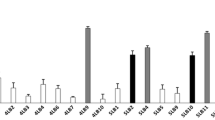
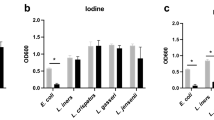
The growth speed was L. gasseri > L. jensenii > L. crispatus > L. iners. Bacterial counts of all Lactobacilli in MRS medium began to decrease at the middle of the log-phase of the growth curve. In addition, L. iners grew to 106 CFU/mL and the others grew to 108 CFU/mL. L. iners was mostly Gram-negative with very short rod, while the others were mostly Gram-positive rods. L. iners was completely killed in the pH 3 medium, however, the others grew (in pH 3 medium) in 1/100 order compared with those in the pH 6 medium.
Conclusion
L. iners was not a typical gram-positive long rod Lactobacilli and presented weak acid-resistance. The reasons why L. iners is detected in abnormal vaginal microbial flora were presumed to be due to the unique morphologic and microbiologic characteristics.





Similar content being viewed by others
References
Yoshimura K, Morotomi N, Fukuda K, Nakano M, Kashimura M, Hachisuga T, Taniguchi H (2011) Intravaginal microbial flora by the 16S rRNA gene sequencing. Am J Obstet Gynecol 205(3):235 e231–239. https://doi.org/10.1016/j.ajog.2011.04.018
Catallozzi M, Fraiz LD, Hargreaves KM, Zimet GD, Stanberry LR, Ratner AJ, Gelber SE, Rosenthal SL (2017) Pregnant women's attitudes about topical microbicides for the prevention and treatment of bacterial vaginosis during pregnancy. Int J STD AIDS 28(9):881–886. https://doi.org/10.1177/0956462416679067
Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29(2):297–301
Kiss H, Petricevic L, Husslein P (2004) Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 329(7462):371. https://doi.org/10.1136/bmj.38169.519653.EB
Mohammadzadeh F, Dolatian M, Jorjani M, Alavi Majd H (2014) Diagnostic value of Amsel's clinical criteria for diagnosis of bacterial vaginosis. Glob J Health Sci 7(3):8–14. https://doi.org/10.5539/gjhs.v7n3p8
Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH (2007) Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol 45(3):1016–1018. https://doi.org/10.1128/JCM.02085-06
Jakobsson T, Forsum U (2007) Lactobacillus iners: a marker of changes in the vaginal flora? J Clin Microbiol 45(9):3145. https://doi.org/10.1128/JCM.00558-07
Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R (2011) The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 118(5):533–549. https://doi.org/10.1111/j.1471-0528.2010.02840.x
Petrova MI, Reid G, Vaneechoutte M, Lebeer S (2017) Lactobacillus iners: Friend or Foe? Trends Microbiol 25(3):182–191. https://doi.org/10.1016/j.tim.2016.11.007
Döderlein A (1892) Das Scheidensekret und seine Bedeutung für das Puerperalfieber. Besold, Leipzig
Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Van Simaey L, De Ganck C, De Backer E, Temmerman M, Vaneechoutte M (2005) Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora. BMC Microbiol 5:61. https://doi.org/10.1186/1471-2180-5-61
Fredricks DN, Fiedler TL, Marrazzo JM (2005) Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353(18):1899–1911. https://doi.org/10.1056/NEJMoa043802
Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G (2002) Vaginal lactobacillus flora of healthy Swedish women. J Clin Microbiol 40(8):2746–2749. https://doi.org/10.1128/jcm.40.8.2746-2749.2002
Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, Schuren FH, van de Wijgert JH (2014) Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 8(9):1781–1793. https://doi.org/10.1038/ismej.2014.26
Falsen E, Pascual C, Sjoden B, Ohlen M, Collins MD (1999) Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol 49(Pt 1):217–221. https://doi.org/10.1099/00207713-49-1-217
De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, Temmerman M, Vaneechoutte M (2007) Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol 7:115. https://doi.org/10.1186/1471-2180-7-115
Funding
The author received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
KY: project development, data collection, and manuscript writing/editing; MO and MS: project development. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KY and MO. The first draft of the manuscript was written by KY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no conflict of interest.
Human and animal rights
This paper does not contain any studies with human nor animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshimura, K., Ogawa, M. & Saito, M. In vitro characteristics of intravaginal Lactobacilli; why is L. iners detected in abnormal vaginal microbial flora?. Arch Gynecol Obstet 302, 671–677 (2020). https://doi.org/10.1007/s00404-020-05634-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05634-y