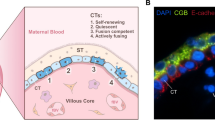
Abstract
Purpose
Pregnancy-associated breast cancer (PABC) is usually diagnosed at an advanced stage in comparison to non-pregnant women. The placenta secretes hormones and cytokines, which affect breast cancer progression. Previously, we demonstrated that human placental secretome facilitates the survival and migration of ERα+ breast cancer cells (BCCL), but pregnant women have a relatively high frequency of ERα-negative tumors. In the current study, we analyzed the effect of placental secretome on ERα-negative BCCL.
Methods
BCCL [MCF-7(estrogen/progesterone receptor positive (ERα+/PR+), ERα reduced MCF-7 (siRNA, MCF-7 ERα−), HS-578 and BT-549 cells (both ER−/PR−)] were exposed to supernatants (collected from first trimester human placental explants and from control BCCL) or to E2 + P4 (estrogen + progesterone) in placental supernatant concentrations and then tested for cell proliferation (number, cell cycle, PCNA), cell-death, cell migration, STAT3 pathway activation and functionality.
Results
Silencing ERα in the MCF-7 cells negated the placental supernatant and E2 + P4 enhancement of cell migration (> 130%, p < 0.05), number (> 120%) and survival (~ 130%). However, it had no such effect on MCF-7-ER− migration, which was still elevated in the presence of placental secretome. ER−/PR− BCCL were unaffected by the hormones, but placental secretome significantly elevated their migration (115%), number (140–170%), STAT3 phosphorylation (~ 180%) and BT-549 STAT3 level. These effects were negated by the STAT3 inhibitor.
Conclusions
Placental supernatant facilitates BCCL malignant characteristics by activating ERα in estrogen responsive cells and STAT3 in ERα- BCCL. This indicates a possible mechanism that may underlie PABC’s advanced state and suggests STAT3 pathway as a therapeutic target for PABC.




Similar content being viewed by others
References
Azim HA Jr, Botteri E, Renne G, Dell'orto P, Rotmensz N, Gentilini O, Sangalli C, Pruneri G, Di Nubila B, Locatelli M, Sotiriou C, Piccart M, Goldhirsch A, Viale G, Peccatori FA (2012) The biological features and prognosis of breast cancer diagnosed during pregnancy: a case–control study. Acta Oncol. 51:653–661
Madaras L, Kovacs KA, Szasz AM, Kenessey I, Tokes AM, Szekely B, Baranyak Z, Kiss O, Dank M, Kulka J (2014) Clinicopathological features and prognosis of pregnancy associated breast cancer - a matched case control study. POR 20:581–590
Azim HA Jr, Brohee S, Peccatori FA, Desmedt C, Loi S, Lambrechts D, Dell'Orto P, Majjaj S, Jose V, Rotmensz N, Ignatiadis M, Pruneri G, Piccart M, Viale G, Sotiriou C (2014) Biology of breast cancer during pregnancy using genomic profiling. Endocr Relat Cancer 21:545–554
Azim HA Jr, Partridge AH (2014) Biology of breast cancer in young women. Breast Cancer Res 16:427
Azim HA Jr, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, Haibe-Kains B, Piccart MJ, Sotiriou C, Loi S (2012) Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 18:1341–1351
Aboagye-Mathiesen G, Toth FD, Zdravkovic M, Ebbesen P (1994) Production of interferons in human placental trophoblast subpopulations and their possible roles in pregnancy. Clin Diagn Lab Immunol 1:650–659
Liu SH, Huang JP, Lee RK, Huang MC, Wu YH, Chen CY, Chen CP (2010) Paracrine factors from human placental multipotent mesenchymal stromal cells protect endothelium from oxidative injury via STAT3 and manganese superoxide dismutase activation. Biol Reprod 82:905–913
Epstein Shochet G, Tartakover Matalon S, Drucker L, Pomeranz M, Fishman A, Rashid G, Oron-Karni V, Pasmanik-Chor M, Lishner M (2011) Hormone-dependent placental manipulation of breast cancer cell migration. Hum Reprod
Epstein Shochet G, Drucker L, Pasmanik-Chor M, Pomeranz M, Fishman A, Tartakover Matalon S, Lishner M (2015) First trimester human placental factors induce breast cancer cell autophagy. Breast Cancer Res Treat 149:645–654
Guarneri V, Conte P (2009) Metastatic breast cancer: therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist 14:645–656
Epstein Shochet G, Tartakover Matalon S, Drucker L, Pomeranz M, Fishman A, Rashid G, Oron-Karni V, Pasmanik-Chor M, Lishner M (2012) Hormone-dependent placental manipulation of breast cancer cell migration. Hum Reprod (Oxford, England) 27:73–88
Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 9:1603–1614
McDaniel JM, Varley KE, Gertz J, Savic DS, Roberts BS, Bailey SK, Shevde LA, Ramaker RC, Lasseigne BN, Kirby MK, Newberry KM, Partridge EC, Jones AL, Boone B, Levy SE, Oliver PG, Sexton KC, Grizzle WE, Forero A, Buchsbaum DJ, Cooper SJ, Myers RM (2016) Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget
Epstein Shochet G, Tartakover-Matalon S, Drucker L, Pasmanik-Chor M, Pomeranz M, Fishman A, Lishner M (2014) Placenta-breast cancer cell interactions promote cancer cell epithelial mesenchymal transition via TGFbeta/JNK pathway. Clin Exp Metas 31:961–975
Tartakover-Matalon S, Mizrahi A, Epstein G, Shneifi A, Drucker L, Pomeranz M, Fishman A, Radnay J, Lishner M (2010) Breast cancer characteristics are modified by first trimester human placenta: in vitro co-culture study. Hum Reprod (Oxford, England) 25:2441–2454
Fernandopulle SM, Cher-Siangang P, Tan PH (2006) Breast carcinoma in women 35 years and younger: a pathological study. Pathology 38:219–222
Shousha S (2000) Breast carcinoma presenting during or shortly after pregnancy and lactation. Arch Pathol Lab Med 124:1053–1060
Banerjee K, Resat H (2016) Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer 138:2570–2578
McDaniel JM, Varley KE, Gertz J, Savic DS, Roberts BS, Bailey SK, Shevde LA, Ramaker RC, Lasseigne BN, Kirby MK, Newberry KM, Partridge EC, Jones AL, Boone B, Levy SE, Oliver PG, Sexton KC, Grizzle WE, Forero A, Buchsbaum DJ, Cooper SJ, Myers RM (2017) Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget 8:8226–8238
Xiong A, Yang Z, Shen Y, Zhou J, Shen Q (2014) Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers 6:926–957
Wang JP, Hielscher A (2017) Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer 8:674–682
Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW (2013) Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J 27:2553–2563
Shochet GE, Komemi O, Sadeh-Mestechkin D, Pomeranz M, Fishman A, Drucker L, Lishner M, Matalon ST (2016) Heat shock protein-27 (HSP27) regulates STAT3 and eIF4G levels in first trimester human placenta. J Mol Histol 47:555–563
Bowen JM, Chamley L, Mitchell MD, Keelan JA (2002) Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta 23:239–256
Kauma SW, Herman K, Wang Y, Walsh SW (1993) Differential mRNA expression and production of interleukin-6 in placental trophoblast and villous core compartments. Am J Reprod Immunol. 30:131–135
Acknowledgements
This work constitutes a portion of the M.Sc. thesis of Michal Bar, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. We thank Thom Rofe for the English editing.
Funding
This study was supported by the Dr. Leo Mintz Foundation, the internal funds of Sackler Faculty of Medicine, Tel Aviv University, Israel.
Author information
Authors and Affiliations
Contributions
MB: data collection and management, data analysis, manuscript review and editing. OK: placentae collection, manuscript review and editing. MP: placentae collection, manuscript review and editing. AF: placentae collection, manuscript review and editing. LD: project development, analysis and manuscript review and editing. STM: protocol and project development, data analysis, and manuscript writing. ML: project development, analysis and manuscript review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All the procedures performed in this study were in accordance with the ethical standards of the ethics Helsinki committee of Meir Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bar, M., Komemi, O., Pomeranz, M. et al. Placental supernatants’ enhancement of the metastatic potential of breast cancer cells: is estrogen receptor (ERα) essential for this phenomenon?. Arch Gynecol Obstet 300, 981–991 (2019). https://doi.org/10.1007/s00404-019-05243-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05243-4