Abstract
Purpose
Polycystic ovary syndrome (PCOS) is an important disease that may alter metabolic balances of the whole body. Progranulin is a growth factor which is related to epithelial, neuronal growth and oogenesis. Here, we aimed to investigate the diagnostic value of the levels of Progranulin in the clinical setting of PCOS, and its metabolic effects.
Methods
Forty-one adolescents and young women with PCOS and 39 age and body mass index matched adolescents and young women as a control group who attended to the youth center of a tertiary referral center were included in this cross-sectional case–control study. Progranulin levels, indices of insulin sensitivity, lipidemic markers, metabolic syndrome (MetS) criteria were compared between the groups.
Results
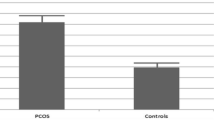
Progranulin levels in patients with PCOS (7.48 ± 1.93 ng/mL) were significantly higher than in the control group (6.25 ± 1.98 ng/mL) (p = 0.006). Luteinizing hormone (LH) levels, LH/Follicle stimulating hormone (FSH) ratios, free testosterone, dehydroepiandrosterone sulfate (DHEAS), C-reactive protein (CRP) levels in patients with PCOS were significantly higher than in the control group (p < 0.05, for all). The MetS was present in 8 (19.5 %) of the patients in the study group and in 1 (2.3 %) of the patients in the control group (p = 0.029). There was significant inverse correlation between high-density lipoprotein cholesterol (HDL-C) and progranulin levels of patients diagnosed with PCOS (p = 0.008).
Conclusions
Progranulin may be a novel biomarker for cardiovascular risk in patients with PCOS, thus these cases should be directed to close follow-up for possible cardiovascular diseases. Future larger studies should focus on this entity.
Similar content being viewed by others
References
Azziz R, Carmina E, Dewailly D et al (2009) The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488
Nestler JE, Jakubowicz DJ, de Vargas AF et al (1998) Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83:2001–2005
Diamanti- Kandarakis E, Papavassiliou AG, Kandarakis SA et al (2007) Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab 18:280–285
Ryan CL, Baranowski DC, Chitramuthu BP et al (2009) Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci 10:130
Suzuki M, Matsumuro M, Hirabayashi K et al (2000) Oocyte-specific expression of granulin precursor (acrogranin) in rat ovary. J Reprod Dev 46:271–277
Fauser BC, Tarlatzis BC, Rebar RW et al (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97(28–38):e25
Yildiz BO, Bolour S, Woods K, Moore A, Azziz R (2010) Visually scoring hirsutism. Hum Reprod Update 16:51–64
Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR (2009) Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol 25:335–343
Cook S, Auinger P, Li C et al (2008) Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr 152:165–170
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Celik Y (2007) Biostatistics, principles of research, 2nd edn. Dicle University Press, Diyarbakir
Baba T, Hoff HB 3rd, Nemoto H et al (1993) Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev 34:233–243
He Z, Ong CHP, Halper J, Bateman A (2003) Progranulin is a mediator of the wound response. Nat Med 9:225–229
Brown Jones M, Michener CM, Blanchette JO et al (2003) The granulin-epithelin precursor/PC-cell-derived growth factor is a growth factor for epithelial ovarian cancer. Clin Cancer Res 9:44–51
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA (1998) Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 101:2622–2629
Wang J, Van Damme P, Cruchaga C et al (2010) Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem 112:1305–1315
Hsueh AJ, Kawamura K, Cheng Y, Fauser BC (2015) Intraovarian control of early folliculogenesis. Endocr Rev 36:1–24
Wild RA, Rizzo M, Clifton S, Carmina E (2011) Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 95(1073–9):e1–e11
Glueck CJ, Morrison JA, Goldenberg N, Wang P (2009) Coronary heart disease risk factors in adult premenopausal white women with polycystic ovary syndrome compared with a healthy female population. Metabolism 58:714–721
Legro RS, Kunselman AR, Dunaif A (2001) Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111:607–613
Essah PA, Nestler JE, Carmina E (2008) Differences in dyslipidemia between American and Italian women with polycystic ovary syndrome. J Endocrinol Invest 31:35
Barter P, Gotto AM, LaRosa JC et al (2007) HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357:1301–1310
Silfen ME, Denburg MR, Manibo AM et al (2003) Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 88:4682–4688
Berenson GS, Srinivasan SR, Bao W et al (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338:1650–1656
Toulis KA, Goulis DG, Mintziori G et al (2011) Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 17:741–760
Türkçüoğlu I, Kafkasli A, Meydanli MM, Ozyalin F, Taşkapan C (2011) Independent predictors of cardiovascular risk in polycystic ovarian syndrome. Gynecol Endocrinol 27:915–919
Cheng Q, Xia W, Yang S et al (2013) Association of serum pigment epithelium-derived factor with high-sensitivity C-reactive protein in women with polycystic ovary syndrome. J Endocrinol Invest 36:632–635
Keskin Kurt R, Okyay AG, Hakverdi AU et al (2014) The effect of obesity on inflammatory markers in patients with PCOS: a BMI-matched case-control study. Arch Gynecol Obstet 290:315–319
Okura H, Yamashita S, Ohama T et al (2010) HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J Atheroscler Thromb. 17:568–577
Matsubara T, Mita A, Minami K et al (2012) PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab 15:38–50
Bayram F, Kocer D, Ozsan M, Muhtaroglu S (2012) Evaluation of endothelial dysfunction, lipid metabolism in women with polycystic ovary syndrome: relationship of paraoxonase 1 activity, malondialdehyde levels, low-density lipoprotein subfractions, and endothelial dysfunction. Gynecol Endocrinol 28:497–501
Köninger A, Koch L, Edimiris P et al (2014) Anti-Mullerian Hormone: an indicator for the severity of polycystic ovarian syndrome. Arch Gynecol Obstet 290:1023–1030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ersoy, A.O., Tokmak, A., Ozler, S. et al. Are progranulin levels associated with polycystic ovary syndrome and its possible metabolic effects in adolescents and young women?. Arch Gynecol Obstet 294, 403–409 (2016). https://doi.org/10.1007/s00404-016-4096-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4096-8