Abstract
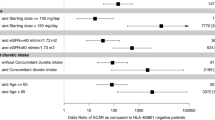
Human leukocyte antigen (HLA)-B*58:01 allele is a significant risk factor for allopurinol-induced severe cutaneous adverse reactions (SCARs) which is potentially fatal. In some studies, chronic kidney disease (CKD) was also implicated to compound the risk of SCARs. We aim to investigate if pre-treatment HLA-B*58:01 screening can prevent allopurinol-induced SCARs in Chinese patients with CKD and its cost-effectiveness. We prospectively recruited Chinese CKD patients who required allopurinol during 2011–2015 and performed pre-treatment HLA testing (HLA screening group). Patients tested positive for HLA-B*58:01 were refrained from allopurinol while those tested negative were prescribed allopurinol. The incidence of SCARs in the HLA screening group was compared with the historical control in previous 5 years and the cost-effectiveness of HLA testing was analyzed. In the historical control (2006–2010), 3605 patients on allopurinol were screened, 22 out of 1027 (2.14%) CKD Chinese patients newly started on allopurinol developed SCARs, including 6 SJS/TEN. In the HLA screening group, 28 out of 192 patients (14.6%) tested HLA-B*58:01 positive were advised to avoid allopurinol; 156 out of 164 HLA-B*58:01-negative patients received allopurinol and none developed SCARs. The incidence rate of SCARs was significantly lower in the HLA screening group compared with controls (0% vs 2.14% respectively, p = 0.037*). The targeted HLA screening approach was associated with lower healthcare costs compared with no HLA screening (US$ 92,430 vs US$ 281,226). Pre-treatment HLA-B*58:01 screening is cost-effective to target on patients with CKD in Chinese to prevent allopurinol-induced SCARs.

Similar content being viewed by others
References
Chung WH, Hung SI, Chen YT (2007) Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol 7:317–323. https://doi.org/10.1097/ACI.0b013e3282370c5f
Hung SI, Chung WH, Liou LB et al (2005) HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 102:4134–4139. https://doi.org/10.1073/pnas.0409500102
Chiu ML, Hu M, Ng MH et al (2012) Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol 167:44–49. https://doi.org/10.1111/j.1365-2133.2012.10894.x
Goncalo M (2018) HLA-B*58:01 is not the only risk factor associated with allopurinol-induced severe cutaneous adverse drug reactions. Ann Transl Med 6:S7. https://doi.org/10.21037/atm.2018.08.42
Klein J, Sato A (2000) The HLA system. First of two parts. N Engl J Med 343:702–709. https://doi.org/10.1056/NEJM200009073431006
Kwok J, Guo M, Yang W et al (2016) HLA-A, -B, -C, and -DRB1 genotyping and haplotype frequencies for a Hong Kong Chinese population of 7595 individuals. Hum Immunol 77:1111–1112. https://doi.org/10.1016/j.humimm.2016.10.005
Chen Z, Liew D, Kwan P (2014) Real-world efficiency of pharmacogenetic screening for carbamazepine-induced severe cutaneous adverse reactions. PLoS ONE 9:e96990. https://doi.org/10.1371/journal.pone.0096990
Ko TM, Tsai CY, Chen SY et al (2015) Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 351:h4848. https://doi.org/10.1136/bmj.h4848
Cheng H, Yan D, Zuo X et al (2018) A retrospective investigation of HLA-B*5801 in hyperuricemia patients in a Han population of China. Pharmacogenet Genomics 28:117–124. https://doi.org/10.1097/FPC.0000000000000334
Chapter 1: Definition and classification of CKD (2013). Kidney Int Suppl (2011) 3:19–62. https://doi.org/10.1038/kisup.2012.64
Cheung NT, Fung V, Wong WN et al (2007) Principles-based medical informatics for success–how Hong Kong built one of the world’s largest integrated longitudinal electronic patient records. Stud Health Technol Inform 129:307–310
Bastuji-Garin S, Rzany B, Stern RS et al (1993) Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 129:92–96
Roujeau JC (1997) Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol 24:726–729. https://doi.org/10.1111/j.1346-8138.1997.tb02524.x
Arellano F, Sacristan JA (1993) Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother 27:337–343. https://doi.org/10.1177/106002809302700317
Roujeau JC, Stern RS (1994) Severe adverse cutaneous reactions to drugs. N Engl J Med 331:1272–1285. https://doi.org/10.1056/NEJM199411103311906
Choudhary S, McLeod M, Torchia D, Romanelli P (2013) Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol 6:31–37
Sassolas B, Haddad C, Mockenhaupt M et al (2010) ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 88:60–68. https://doi.org/10.1038/clpt.2009.252
Jung JW, Song WJ, Kim YS et al (2011) HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 26:3567–3572. https://doi.org/10.1093/ndt/gfr060
Huang HY, Luo XQ, Chan LS et al (2011) Cutaneous adverse drug reactions in a hospital-based Chinese population. Clin Exp Dermatol 36:135–141. https://doi.org/10.1111/j.1365-2230.2010.03922.x
Rzany B, Mockenhaupt M, Baur S et al (1996) Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol 49:769–773. https://doi.org/10.1016/0895-4356(96)00035-2
Roujeau JC, Kelly JP, Naldi L et al (1995) Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 333:1600–1607. https://doi.org/10.1056/NEJM199512143332404
Harris V, Jackson C, Cooper A (2016) Review of toxic epidermal necrolysis. Int J Mol Sci 17:2135. https://doi.org/10.3390/ijms17122135
Su SC, Chung WH (2013) Update on pathobiology in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol Sin 31:175–180
Atzori L, Pinna AL, Mantovani L et al (2012) Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department–Cagliari University (Italy). J Eur Acad Dermatol Venereol 26:1424–1430. https://doi.org/10.1111/j.1468-3083.2011.04313.x
Lam MP, Yeung CK, Cheung BM (2013) Pharmacogenetics of allopurinol–making an old drug safer. J Clin Pharmacol 53:675–679. https://doi.org/10.1002/jcph.67
Suzuki Y, Inagi R, Aono T et al (1998) Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol 134:1108–1112. https://doi.org/10.1001/archderm.134.9.1108
Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R (2001) Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis 60:981–983. https://doi.org/10.1136/ard.60.10.981
Ng CY, Yeh YT, Wang CW et al (2016) Impact of the HLA-B(*)58:01 allele and renal impairment on allopurinol-induced cutaneous adverse reactions. J Invest Dermatol 136:1373–1381. https://doi.org/10.1016/j.jid.2016.02.808
Cao ZH, Wei ZY, Zhu QY et al (2012) HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics 13:1193–1201. https://doi.org/10.2217/pgs.12.89
Somkrua R, Eickman EE, Saokaew S et al (2011) Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet 12:118. https://doi.org/10.1186/1471-2350-12-118
Yang F, Yang Y, Zhu Q et al (2015) Research on susceptible genes and immunological pathogenesis of cutaneous adverse drug reactions in Chinese hans. J Investig Dermatol Symp Proc 17:29–31. https://doi.org/10.1038/jidsymp.2015.6
Park HJ, Kim YJ, Kim DH et al (2016) HLA allele frequencies in 5802 Koreans: varied allele types associated with SJS/TEN according to culprit drugs. Yonsei Med J 57:118–126. https://doi.org/10.3349/ymj.2016.57.1.118
Goncalo M, Coutinho I, Teixeira V et al (2013) HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol 169:660–665. https://doi.org/10.1111/bjd.12389
Lonjou C, Borot N, Sekula P et al (2008) A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 18:99–107. https://doi.org/10.1097/FPC.0b013e3282f3ef9c
Genin E, Schumacher M, Roujeau JC et al (2011) Genome-wide association study of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe. Orphanet J Rare Dis 6:52. https://doi.org/10.1186/1750-1172-6-52
Wu R, Cheng YJ, Zhu LL et al (2016) Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies. Oncotarget 7:81870–81879. https://doi.org/10.18632/oncotarget.13250
Kang HR, Jee YK, Kim YS et al (2011) Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics 21:303–307. https://doi.org/10.1097/FPC.0b013e32834282b8
Ke CH, Chung WH, Wen YH et al (2017) Cost-effectiveness analysis for genotyping before allopurinol treatment to prevent severe cutaneous adverse drug reactions. J Rheumatol 44:835–843. https://doi.org/10.3899/jrheum.151476
Park DJ, Kang JH, Lee JW et al (2015) Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res (Hoboken) 67:280–287. https://doi.org/10.1002/acr.22409
Park HW, Kim DK, Kim SH et al (2019) Efficacy of the HLA-B(*)58:01 screening test in preventing allopurinol-induced severe cutaneous adverse reactions in patients with chronic renal insufficiency-a prospective study. J Allergy Clin Immunol Pract 7:1271–1276. https://doi.org/10.1016/j.jaip.2018.12.012
Khanna D, Fitzgerald JD, Khanna PP et al (2012) 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 64:1431–1446. https://doi.org/10.1002/acr.21772
Saito Y, Stamp LK, Caudle KE et al (2016) Clinical Pharmacogenetics implementation consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharmacol Ther 99:36–37. https://doi.org/10.1002/cpt.161
Acknowledgements
The authors would like to extend the appreciation to Dr. Kendrick Co Shih who reviewed and advised on SJS/TEN cases with ocular complications in the study.
Funding
This project was funded by the S.K. Yee Medical Foundation (Project number 212218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no competing conflict of interest from all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong, C.SM., Yeung, CK., Chan, CY. et al. HLA-B*58:01 screening to prevent allopurinol-induced severe cutaneous adverse reactions in Chinese patients with chronic kidney disease. Arch Dermatol Res 314, 651–659 (2022). https://doi.org/10.1007/s00403-021-02258-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-021-02258-3