Abstract
Introduction
Fibrin glues are currently used by surgeons and can facilitate the handling of biomaterials. Combining fibrin glue with calcium phosphate bioceramics gives a mouldable composite that cements the granules into the implantation site. In addition to the mechanical aspect of the composite, it has been suggested that the mixture also promotes wound healing. These human blood derivatives contain natural (aprotinin) or synthetic (tranexamic acid) antifibrinolytic substances. We compared the bioactivity of two composites combining calcium phosphate granules with two different types of fibrin glue, one with aprotinin and the other with tranexamic acid.
Materials and methods
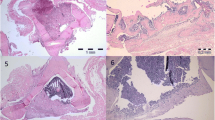
The composite was composed of fibrin glue (Tissucol) and 1 to 2 mm granules of biphasic calcium phosphate granules (MBCP) with a volume ratio of 1 for 2. Bone cavities were drilled in 12 New Zealand rabbits and filled with a composite with aprotinin-fibrin glue on the right condyle and one with tranexamic acid-fibrin glue on the left condyle. The rabbits were randomized into two groups: 3 and 6 weeks of delay. Light microscopy, scanning electron microscopy and image analysis were performed.
Results
No adverse reactions were observed in either sample. Bony ingrowth associated with bioceramic resorption by osteoclastic TRAP-positive cells was noted. No significant difference was observed between the two composites. The bony ingrowth and ceramic resorption were qualitatively and quantitatively similar with both composites.
Conclusion
This study demonstrated that the choice of a natural (aprotinin) or synthetic (tranexamic acid) antifibrinolytic agent in the fibrin sealant associated with calcium phosphate granules and used as a bone substitute had no effect on the bioactivity of the composite. It remained efficient in bone reconstruction, no adverse effects were observed, and the bony ingrowth was qualitatively and quantitatively equivalent with the two types of fibrin sealant.




Similar content being viewed by others
References
Alving BM, Weinstein MJ, Finlayson JS, Menitove JE, Fratantoni JC (1995) Fibrin sealant: summary of a conference on characteristics and clinical uses [see comments]. Transfusion 35:783-790
Arbes H, Bosch P, Lintner F, Salzer M (1981) First clinical experience with heterologous cancellous bone grafting combined with the fibrin adhesive system (F.A.S.). Arch Orthop Trauma Surg 98:183–188
Athanasou NA, Quinn J (1990) Immmunophenotypic differences between osteoclasts and macrophage polykaryons: immunological distinction and implications for osteoclast ontogeny and function. J Clin Pathol 43:997–1003
Basle MF, Chappard D, Grizon F, Filmon R, Delecrin J, Daculsi G, Rebel A (1993) Osteoclastic resorption of Ca-P biomaterials implanted in rabbit bone. Calcif Tissue Int 53:348–356
Beduschi R, Beduschi MC, Wojno KJ, Jhung M, Williams AL, Wolf JS Jr (1999) Antifibrinolytic additives to fibrin glue for laparoscopic wound closure in urinary tract. J Endourol 13:283–287
Bertrand B, Doyen A, Eloy P (1996) Triosite implants and fibrin glue in the treatment of atrophic rhinitis: technique and results. Laryngoscope 106:652–657
Bosch P, Braun F, Spangler HP (1977) The technique of fibrin glue in cancellous bone transplants [in German]. Arch Orthop Unfallchir 90:63–75
Bosch P, Lintner F, Arbes H, Brand G (1980) Experimental investigations of the effect of the fibrin adhesive on the Kiel heterologous bone graft. Arch Orthop Trauma Surg 96:177–185
Brittberg M, Sjogren-Jansson E, Lindahl A, Peterson L (1997) Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials 18:235–242
Byrne DJ, Hardy J, Wood RA, McIntosh R, Cuschieri A (1991) Effect of fibrin glues on the mechanical properties of healing wounds. Br J Surg 78:841–843
Daculsi G, Bagot d’Arc M, Corlieu P, Gersdorff M (1992) Macroporous biphasic calcium phosphate efficiency in mastoid cavity obliteration: experimental and clinical findings. Ann Otol Rhinol Laryngol 101:669–674
Daculsi G, Passuti N, Martin S, Deudon C, Legeros RZ (1990) Macroporous calcium phosphate ceramic for long bone surgery in human and dogs. Clinical and histological study. J Biomed Mater Res 24:379–396
Dietrich W, Spath P, Ebell A, Richter JA (1997) Prevalence of anaphylactic reactions to aprotinin: analysis of two hundred forty-eight reexposures to aprotinin in heart operations. J Thorac Cardiovasc Surg 113:194–201
Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G (1998) Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 19:133–139
Gauthier O, Bouler JM, Weiss P, Bosco J, Aguado E, Daculsi G (1999) Short-term effects of mineral particle sizes on cellular degradation activity after implantation of injectable calcium phosphate biomaterials and the consequences for bone substitution. Bone 25:71–74
Gauthier O, M. BJ, Weiss P, Bosco J, Daculsi G, Aguado E (1999) Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J Biomed Mater Res 47:28–35
Gerngross H, Burri C, Claes L (1986) Experimental studies on the influence of fibrin adhesive, factor XIII, and calcitonin on the incorporation and remodeling of autologous bone grafts. Arch Orthop Trauma Surg 106:23–31
Gersdorff M, Vilain J (1989) Osseous reconstruction in microsurgery of the middle ear. Acta Otorhinolaryngol Belg 43:481–488
Jarzem P, Harvey EJ, Shenker R, Hajipavlou A (1996) The effect of fibrin sealant on spinal fusions using allograft in dogs. Spine 21:1307–1312
Jorgensen PH, Jensen KH, Andreassen TT (1987) Mechanical strength in rat skin incisional wounds treated with fibrin sealant. J Surg Res 42:237–241
Kallis P, Tooze JA, Talbot S, Cowans D, Bevan DH, Treasure T (1994) Aprotinin inhibits fibrinolysis, improves platelet adhesion and reduces blood loss. Results of a double-blind randomized clinical trial. Eur J Cardiothorac Surg 8:315–323
Kania RE, Meunier A, Hamadouche M, Sedel L, Petite H (1998) Addition of fibrin sealant to ceramic promotes bone repair: long-term study in rabbit femoral defect model. J Biomed Mater Res 43:38–45
Kjaergard HK, Weis-Fogh US (1994) Important factors influencing the strength of autologous fibrin glue; the fibrin concentration and reaction time--comparison of strength with commercial fibrin glue. Eur Surg Res 26:273–276
Knighton DR, Hunt TK, Thakral KK, Goodson WH (1982) Role of platelets and fibrin in the healing sequence. An in vivo study of angiogenesis and collagen synthesis. Ann Surg 193:379
Kon NF, Masumo H, Nakajima S, Tozawa R, Kimura M, Maeda S (1994) Anaphylactic reaction to aprotinin following topical use of biological tissue sealant. Masui 43:1606–1610
Lerner R, Binur NS (1990) Current status of surgical adhesives. J Surg Res 48:165–181
Martinowitz U, Saltz R (1996) Fibrin sealant. Curr Opin Hematol 3:395–402
Martinowitz U, Schulman S (1995) Fibrin sealant in surgery of patients with a hemorrhagic diathesis. Thromb Haemost 74:486–492
Martinowitz U, Spotnitz WD (1997) Fibrin tissue adhesives. Thromb Haemost 78:661–666
Matras H (1985) Fibrin sealant in maxillofacial surgery. Development and indications. A review of the past 12 years. Facial Plast Surg 2:297–313
Meijer HJ, Steen WH, Bosman F, Wittkampf AR (1997) Radiographic evaluation of mandibular augmentation with prefabricated hydroxylapatite/fibrin glue implants [see comments]. J Oral Maxillofac Surg 55:138–144; discussion 144–145
Mitsuhata H, Horiguchi Y, Saitoh J, Saitoh K, Fukuda H, Hirabayasi Y, Togashi H, Shimizu R (1994) An anaphylactic reaction to topical fibrin glue. Anesthesiology 81:1074–1077
Ono K, Yamamuro T, Nakamura T, Kakutani Y, Kitsugi T, Hyakuna K, Kokubo T, Oka M, Kotoura Y (1988) Apatite-wollastonite containing glass ceramic-fibrin mixture as a bone defect filler. J Biomed Mater Res 22:869–885
Ono K, Yamamuro T, Nakamura T, Kokubo T (1990) Apatite-wollastonite containing glass ceramic granule-fibrin mixture as a bone graft filler: use with low granular density. J Biomed Mater Res 24:11–20
Orsel I, Guillaume A, Feiss P (1997) Anaphylactic shock caused by fibrin glue. Ann Fr Anesth Reanim 16:292–293
Pasquier G, Flautre B, Blary MC, Anselme K, Hardouin P (1996) Injectable percutaneous bone materials: an experimental study in a rabbit model. J Mater Sci Mater Med 7:683–690
Pipan CM, Glasheen WP, Matthew TL, Gonias SL, Hwang LJ, Jane JA, Spotnitz WD (1992) Effects of antifibrinolytic agents on the life span of fibrin sealant. J Surg Res 53:402–407
Radosevich M, Goubran HI, Burnouf T (1997) Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang 72:133–143
Rakocz M, Mazar A, Varon D, Spierer S, Blinder D, Martinowitz U (1993) Dental extractions in patients with bleeding disorders. The use of fibrin glue. Oral Surg Oral Med Oral Pathol 75:280–282
Redl H, Schlag G, Dinges HP (1982) Methods of fibrin seal application. Thorac Cardiovasc Surg 30:223–227
Saltz R, Dimick A, Harris C, Grotting JC, Psillakis J, Vasconez LO (1989) Application of autologous fibrin glue in burn wounds. J Burn Care Rehabil 10:504–507
Scheule AM, Beierlein W, Lorenz H, Ziemer G (1998) Repeated anaphylactic reactions to aprotinin in fibrin sealant. Gastrointest Endosc 48:83–85
Scheule AM, Beierlein W, Wendel HP, Eckstein FS, Heinemann MK, Ziemer G (1998) Fibrin sealant, aprotinin, and immune response in children undergoing operations for congenital heart disease. J Thorac Cardiovasc Surg 115:883–889
Scheule AM, Beierlein W, Wendel HP, Jurmann MJ, Eckstein FS, Ziemer G (1999) Aprotinin in fibrin tissue adhesives induces specific antibody response and increases antibody response of high-dose intravenous application. J Thorac Cardiovasc Surg 118:348–353
Seelich T (1982) Tissucol (Immuno, Vienna): biochemistry and methods of application. J Head Neck Pathol 3:65
Siedentop KH, Park JJ, Sanchez B (1995) An autologous fibrin tissue adhesive with greater bonding power. Arch Otolaryngol Head Neck Surg 121:769–772
Sierra DH (1993) Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J Biomater Appl 7:309–352
Sierra DH, Nissen AJ, Welch J (1990) The use of fibrin glue in intracranial procedures: preliminary results. Laryngoscope 100:360–363
Sirieix D, Chemla E, Castier Y, Massonnet-Castel S, Fabiani JN, Baron JF (1998) Comparative study of different biological glues in an experimental model of surgical bleeding in anesthetized rats: platelet-rich and -poor plasma-based glue with and without aprotinin versus commercial fibrinogen-based glue. Ann Vasc Surg 12:311–316
Turgut M, Erkus M, Tavus N (1999) The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: an experimental study with cats. Acta Neurochir 141:273–278
Wittkampf AR (1989) Fibrin glue as cement for HA-granules. J Craniomaxillofac Surg 17:179–181
Yamada S, Heymann D, Bouler J-M, Daculsi G (1997) Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/B-tricalcium phosphate ratios. Biomaterials 18:1037–1041
Acknowledgements
We would particularly like to thank Biomatlante France (Chantal Gobin) and Baxter Biosciences Biosurgery, Vienna, Austria (Dr K. Bittner and Dr R. Spaethe) for their support of this study and for having supplied the calcium phosphate granules and various fibrin glues. This study was conducted with the financial contribution of the CPER Pays de Loire Biomaterials 2000–2006 and RNTS 2002 from French Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jegoux, F., Goyenvalle, E., Bagot D’arc, M. et al. In vivo biological performance of composites combining micro-macroporous biphasic calcium phosphate granules and fibrin sealant. Arch Orthop Trauma Surg 125, 153–159 (2005). https://doi.org/10.1007/s00402-004-0748-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-004-0748-4