Abstract
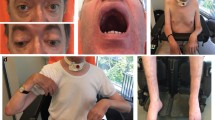
Myotubular myopathy and centronuclear myopathies (CNM) are congenital myopathies characterized by generalized muscle weakness and mislocalization of muscle fiber nuclei. Genetically distinct forms exist, and mutations in BIN1 were recently identified in autosomal recessive cases (ARCNM). Amphiphysins have been implicated in membrane remodeling in brain and skeletal muscle. Our objective was to decipher the pathogenetic mechanisms underlying different forms of CNM, with a focus on ARCNM cases. In this study, we compare the histopathological features from patients with X-linked, autosomal recessive, and dominant forms, respectively, mutated in myotubularin (MTM1), amphiphysin 2 (BIN1), and dynamin 2 (DNM2). We further characterize the ultrastructural defects in ARCNM muscles. We demonstrate that the two BIN1 isoforms expressed in skeletal muscle possess the phosphoinositide-binding domain and are specifically targeted to the triads close to the DHPR–RYR1 complex. Cardiac isoforms do not contain this domain, suggesting that splicing of BIN1 regulates its specific function in skeletal muscle. Immunofluorescence analyses of muscles from patients with BIN1 mutations reveal aberrations of BIN1 localization and triad organization. These defects are also observed in X-linked and autosomal dominant forms of CNM and in Mtm1 knockout mice. In addition to previously reported implications of BIN1 in cancer as a tumor suppressor, these findings sustain an important role for BIN1 skeletal muscle isoforms in membrane remodeling and organization of the excitation–contraction machinery. We propose that aberrant BIN1 localization and defects in triad structure are part of a common pathogenetic mechanism shared between the three forms of centronuclear myopathies.






Similar content being viewed by others
Abbreviations
- ADCNM:
-
Autosomal dominant centronuclear myopathy
- ARCNM:
-
Autosomal recessive centronuclear myopathy
- BAR:
-
Bin/Amphiphysin/Rvs167
- CNM:
-
Centronuclear myopathy
- KO:
-
Knockout
- NADH-TR:
-
Nicotinamide adenine dinucleotide tetrazolium reductase
- OMIM:
-
Online mendelian inheritance in man
- PIs:
-
Phosphoinositides
- SR:
-
Sarcoplasmic reticulum
- XLMTM:
-
X-linked myotubular myopathy
References
Al-Qusairi L, Weiss N, Toussaint A et al (2009) T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci USA 106:18763–18768
Bevilacqua JA, Bitoun M, Biancalana V et al (2009) “Necklace” fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy. Acta Neuropathol 117:283–291
Bitoun M, Bevilacqua JA, Prudhon B et al (2007) Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol 62:666–670
Bitoun M, Maugenre S, Jeannet PY et al (2005) Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 37:1207–1209
Blondeau F, Laporte J, Bodin S, Superti-Furga G, Payrastre B, Mandel JL (2000) Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum Mol Genet 9:2223–2229
Buj-Bello A, Laugel V, Messaddeq N et al (2002) The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci USA 99:15060–15065
Butler MH, David C, Ochoa GC et al (1997) Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of Ranvier in brain and around T tubules in skeletal muscle. J Cell Biol 137:1355–1367
Carson FL (1997) Histotechnology. ASCP press, Chicago
Claeys KG, Maisonobe T, Bohm J et al (2010) Phenotype of a patient with recessive centronuclear myopathy and a novel BIN1 mutation. Neurology 74:519–521
Denic V, Weissman JS (2007) A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130:663–677
Dowling JJ, Vreede AP, Low SE et al (2009) Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5:e1000372
Elliott K, Sakamuro D, Basu A et al (1999) Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene 18:3564–3573
Engel AG, Franzini-Armstrong C (2004) Myology, basic and clinical. McGraw-Hill, New York
Fernando P, Sandoz JS, Ding W et al (2009) Bin1 SRC homology 3 domain acts as a scaffold for myofiber sarcomere assembly. J Biol Chem 284:27674–27686
Flucher BE, Andrews SB, Daniels MP (1994) Molecular organization of transverse tubule/sarcoplasmic reticulum junctions during development of excitation-contraction coupling in skeletal muscle. Mol Biol Cell 5:1105–1118
Frost A, Unger VM, De Camilli P (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137:191–196
Hong TT, Smyth JW, Gao D et al (2010) BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol 8:e1000312
Jeannet PY, Bassez G, Eymard B et al (2004) Clinical and histologic findings in autosomal centronuclear myopathy. Neurology 62:1484–1490
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129
Jungbluth H, Wallgren-Pettersson C, Laporte J (2008) Centronuclear (myotubular) myopathy. Orphanet J Rare Dis 3:26
Kojima C, Hashimoto A, Yabuta I et al (2004) Regulation of Bin1 SH3 domain binding by phosphoinositides. EMBO J 23:4413–4422
Laporte J, Biancalana V, Tanner SM et al (2000) MTM1 mutations in X-linked myotubular myopathy. Hum Mutat 15:393–409
Laporte J, Hu LJ, Kretz C et al (1996) A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13:175–182
Lee E, Marcucci M, Daniell L et al (2002) Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297:1193–1196
Leprince C, Romero F, Cussac D et al (1997) A new member of the amphiphysin family connecting endocytosis and signal transduction pathways. J Biol Chem 272:15101–15105
Luna LG (1992) Histopathological methods and color atlas of special stains and tissue artifacts. Johnson Printers, Downers Grove
McMahon HT, Gallop JL (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590–596
Muller AJ, Baker JF, DuHadaway JB et al (2003) Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol Cell Biol 23:4295–4306
Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC (2004) Targeted deletion of the suppressor gene bin1/amphiphysin2 accentuates the neoplastic character of transformed mouse fibroblasts. Cancer Biol Ther 3:1236–1242
Nicot AS, Laporte J (2008) Endosomal phosphoinositides and human diseases. Traffic 9:1240–1249
Nicot AS, Toussaint A, Tosch V et al (2007) Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 39:1134–1139
North K (2008) What’s new in congenital myopathies? Neuromuscul Disord 18:433–442
Pelé M, Tiret L, Kessler JL, Blot S, Panthier JJ (2005) SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum Mol Genet 14:1417–1427
Pierson CR, Tomczak K, Agrawal P, Moghadaszadeh B, Beggs AH (2005) X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 64:555–564
Prendergast GCMAJ, Ramalingam A, Chang MY (2009) Bar the door: cancer suppression by amphiphysin-like genes. Biochem Biophys Acta 1795:25–36
Ramjaun AR, McPherson PS (1998) Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J Neurochem 70:2369–2376
Razzaq A, Robinson IM, McMahon HT et al (2001) Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 15:2967–2979
Ren G, Vajjhala P, Lee JS, Winsor B, Munn AL (2006) The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol Mol Biol Rev 70:37–120
Rezniczek GA, Konieczny P, Nikolic B et al (2007) Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J Cell Biol 176:965–977
Romero NB (2010) Centronuclear myopathies: a widening concept. Neuromuscul Disord 20:223–228
Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC (1996) BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat Genet 14:69–77
Shpetner HS, Vallee RB (1989) Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell 59:421–432
Taylor GS, Maehama T, Dixon JE (2000) Inaugural article: myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA 97:8910–8915
Tiret L, Blot S, Kessler JL, Gaillot H, Breen M, Panthier JJ (2003) The cnm locus, a canine homologue of human autosomal forms of centronuclear myopathy, maps to chromosome 2. Hum Genet 113:297–306
Tosch V, Vasli N, Kretz C et al (2010) Novel molecular diagnostic approaches for X-linked centronuclear (myotubular) myopathy reveal intronic mutations. Neuromuscul Disord 20:375–381
Tronchere H, Laporte J, Pendaries C et al (2004) Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem 279:7304–7312
Tsai TC, Horinouchi H, Noguchi S et al (2005) Characterization of MTM1 mutations in 31 Japanese families with myotubular myopathy, including a patient carrying 240 kb deletion in Xq28 without male hypogenitalism. Neuromuscul Disord 15:245–252
Unsworth KE, Mazurkiewicz P, Senf F et al (2007) Dynamin is required for F-actin assembly and pedestal formation by enteropathogenic Escherichia coli (EPEC). Cell Microbiol 9:438–449
Wechsler-Reya RJ, Elliott KJ, Prendergast GC (1998) A role for the putative tumor suppressor Bin1 in muscle cell differentiation. Mol Cell Biol 18:566–575
Acknowledgments
We thank Christine Kretz and the IGBMC imaging center for technical assistance. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), University of Strasbourg, Collège de France, Association Française contre les Myopathies (AFM), Fondation Recherche Médicale (FRM DEQ20071210538), Agence Nationale de la Recherche (ANR-06-MRAR 023, ANR-07-BLAN-0065-03, ANR-08-GENOPAT-005), and the E-rare program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toussaint, A., Cowling, B.S., Hnia, K. et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol 121, 253–266 (2011). https://doi.org/10.1007/s00401-010-0754-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0754-2