Abstract
Cerebral amyloid angiopathy (CAA) is a manifestation of amyloid β-protein (Aβ) accumulation in the elderly as well as in patients with Alzheimer’s disease (AD). Two types of CAA have been noted, based on the type of vasculature in which Aβ is deposited: cerebral capillary amyloid angiopathy (capCAA) and non-capCAA. Non-capCAA is a common form of CAA that consists of Aβ deposited in arteries and arterioles. Recent information on Aβ metabolism in the brain suggests that non-capCAA is associated with Aβ secretion into the cerebrospinal fluid via the perivascular space, whereas capCAA is associated with Aβ removal to blood plasma via the capillary endothelium. Aβ40, a major and relatively soluble Aβ isoform, is deposited predominantly in non-capCAA, and Aβ42, which is insoluble and associated more closely than Aβ40 with AD, is deposited predominantly in capCAA. Studying small areas of microscopic size within a given cortical region, we found an inverse association of capCAA and senile plaques. We also found a relative paucity of tau pathology in the small areas with abundant capCAA compared with the small areas with abundant senile plaques within a cortical region with the same cytoarchitecture. We suppose that both capCAA and senile plaques indicate high Aβ42 in the neuropil but that only Aβ42 in the form of insoluble deposits in senile plaques promotes tau abnormality.
Similar content being viewed by others
Introduction
Cerebral amyloid angiopathy (CAA) is a form of amyloid deposition where the amyloid materials accumulate in association with blood vessels in the brain. CAA occasionally causes cerebral hemorrhage or infarction [20, 35, 36, 46], but, in the majority of cases, CAA is not associated with acute clinical manifestations. CAA frequently occurs in the brain of patients with Alzheimer’s disease (AD). CAA is also seen, though less frequently, in the brain of non-demented, aged subjects as well as of demented patients whose pathology does not meet the criteria for AD. In CAA in both AD and aged subjects, amyloid β-protein (Aβ) is the major component of the vascular amyloid. Studies on transgenic mouse models of AD in which mutant human Aβ precursor protein (APP) is expressed by neurons have shown that CAA similar to that observed in the human brain occurs in association with aging [12, 13]. Nevertheless, significance of CAA with respect to the pathophysiology of AD still remains a matter of controversy [43, 44].
Recently, two types of CAA have been noted: cerebral capillary amyloid angiopathy (capCAA) and non-capCAA [37, 42]. In capCAA, Aβ is deposited in the capillaries in the brain parenchyma. In non-capCAA [42], Aβ is deposited in the parenchymal and leptomeningeal arteries/arterioles. Non-capCAA is the common form of CAA and the term CAA refers to non-capCAA in most previous publications. Some investigators argued for the absence of a relationship between the apolipoprotein E ε4 allele and CAA [45], whereas others reported a significant association of the ε4 allele with capCAA [14, 42].
In AD brain, deposited Aβ consists of multiple molecular species with diverse carboxyl termini. Aβ terminating at valine40 (Aβ40) is the major form of Aβ produced in the brain. Aβ terminating at alanine42 (Aβ42) is a minor form of Aβ in normal brain but predominates over Aβ40 in the senile plaque Aβ deposits in AD. There appears to be general agreement that Aβ42 plays an essential role for AD pathogenesis because of its propensity to aggregate more easily than Aβ40 and its in vitro toxicity to neurons [38]. In non-capCAA, Aβ40 is the major component of vascular amyloid. On the other hand, capCAA is characterized by the deposition of Aβ42 together with or in the absence of Aβ40 deposition [2, 7].
Amyloid β-protein is now considered to be removed from the brain parenchyma, at least in part, to the cerebrospinal fluid through the perivascular space. Therefore, an abundance of CAA, similarly to that of senile plaques, may indicate excessive release of Aβ into the brain parenchyma from neurons [15, 16]. The aim of the present study was to reveal whether parenchymal deposition of Aβ42 or its movement to the capillaries in soluble forms is more intimately associated with tau pathology. We hypothesized that Aβ42 that is deposited in the parenchyma plays a more significant role in a tau abnormality than Aβ42 that remains soluble in the interstitial fluid. We compared the occurrence of tau pathology and senile plaques in a number of cases in two closely located small areas, one rich in capCAA and the other poor in capCAA, within the same cortical layer and region.
Materials and methods
The autopsied brains were fixed in 10% neutral formalin, embedded in paraffin wax, cut into 10 μm thick sections and mounted on glass slides coated with Bioden®. Sections were taken from the frontal, temporal, parietal and occipital cortices. In some cases, small brain blocks were cut at autopsy and fixed in 4% parafolmaldehyde in 0.1 M phosphate buffer, pH 7.4, for 48 h in the cold. Brain blocks were transferred to 15% sucrose in 0.1 M phosphate buffered saline (PBS), pH 7.4, and then cut on a freezing microtome into 30-μm thick sections for free-floating immunohistochemical staining. The diagnosis of each patient was made on the neuropathological basis in our routine clinico-pathological conferences.
We first screened brains of approximately 1,000 patients consecutively autopsied from 1974 to 2004 in the Tokyo Metropolitan Matsuzawa Hospital for cases with abundant CAA. By examination of the occipital cortex (Brodmann’s areas 17–19) stained with Bodian’s or methenamine silver staining, we identified 39 cases with many CAA and immunostained adjacent sections for Aβ. Immunohistochemistry for Aβ revealed 12 of the 39 cases were rich in capCAA. We finally picked up nine sections from eight cases that contained both capCAA and senile plaques abundantly in the same Brodmann’s area. All eight cases met the criteria for definite AD of CERAD [31] and had tau pathology of Braak and Braak stage V or VI [10]. One case also had Lewy body pathology that met the consensus guidelines for dementia with Lewy bodies [30]. Table 1 summarizes the diagnosis, age, sex, and the Braak stage for neurofibrillary tangles (NFT) and senile plaques [10] of the eight patients employed in this study. All sections contained, in the same Brodmann’s area, capCAA of score 4 or higher, amyloid plaques of score 3 or higher and tau pathology of score 4 or higher by our assessment criteria described later in Results (Table 2).
For immunohistochemistry of paraffin-embedded tissues, sections were deparaffinized prior to staining. Both glass-mounted and free-floating tissue sections were pretreated with 100% formic acid for 1 min and then with 0.5% H2O2 for 30 min, followed by incubation with a primary antibody. The primary antibody labeling was detected using the avidin-biotinylated HRP complex (ABC) system (Vector Lab, CA, USA) coupled to a diaminobenzidine (DAB) reaction intensified with nickel ammonium sulfate to yield a purple precipitate. The primary antibodies employed in this study were: AT8 (PHF-tau, mouse monoclonal, Inogenetics, USA), E50 (Aβ17-31, rabbit polyclonal, made in our laboratory), Aβter40 and Aβter42 (both generous gift of Dr. Hiroshi Mori, Osaka City University, School of Medicine) [32, 33]. For double immunostaining, sections were treated with 0.5% H2O2 for 30 min after the first immunohistochemical cycle and then treated with primary antibody of the second cycle. The primary antibody labeling of the second cycle was visualized similarly to the first except that nickel was eliminated to yield a brown precipitate.
For statistical analyses, Prism® version 3.0 (a graph pad software on Windows®) was used.
Results
While occasional capCAA was present among non-capCAA in aged as well as AD subjects, abundant capCAA was found only in a limited number of cases. In such cases, capCAA tended to occur in clusters in cortical layers III–IV (Fig. 1). This contrasts with non-capCAA, which is frequent in the leptomeninges and superficial cortical layers (Fig. 1). A spatial relationship between capCAA and non-capCAA is therefore not evident at the microscopic level, although both CAAs macroscopically show a similar distribution pattern among Brodmann’s areas. In non-capCAA, Aβ40 predominated over Aβ42. In capCAA, on the other hand, Aβ42 was deposited equally to (Fig. 2a) or, more frequently, predominantly over Aβ40 (Fig. 2b), a result that confirms previous reports [2, 7].
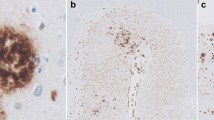
A low power photomicrograph of Aβ (E50, brown) and tau (AT8, purple) double-immunostaining of the visual cortex from a patient with Alzheimer’s disease (AD). Non-capCAA is seen predominantly in the superficial cortical layers. CapCAA is most frequent in cortical layer IV but is also present in other cortical layers (arrows). The inset shows five-times higher power magnification of the boxed part. II, III and IV indicate the cortical layers. Tau pathology is not visible at the low power magnification and is absent in the enlarged, cap-CAA-frequent area. Bar = 200 μm
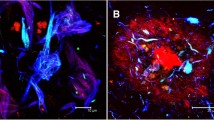
Cerebral capillary amyloid angiopathy doubly-immunostained for Aβ40 (purple) and Aβ42 (brown). a Some capCAA is positive for both Aβ40 and Aβ42, which results in brownish purple labeling. b A significant number of capCAA is Aβ42 positive but Aβ40 negative. In this case, the double staining gives brown labeling. Bar = 25 μm
Figure 3a illustrates Aβ immunostaining of the Brodmann’s area 17 of a case with abundant CAA and senile plaques. In the right half of the visual field, cortical layers III and IV show many capCAA but few senile plaques, while in the left half many senile plaques but no capCAA are present. Tau immunostaining of nearby sections revealed that tau-positive neuropil threads (NT) and dystrophic neurites are frequent in the area with many senile plaques but are significantly fewer in the area with an abundance of capCAA (Fig. 3b). Figure 3c confirms that both senile plaque-rich and capCAA-rich areas are located within the area 17. A similar dissociation between Aβ deposits and tau pathology was seen in all cases examined, and a relative paucity of tau pathology was evident in capCAA-frequent and senile plaque-rare small areas compared with senile plaque-rich small areas within a given cortical region.
a A low power photomicrograph of the primary visual cortex (area 17) of a patient with AD/dementia with Lewy bodies immunostained for Aβ (E50). The left half is rich in senile plaques and poor in capCAA whereas the right half contains many cap-CAA and few senile plaques. The inset shows five-times higher power magnification of the boxed part. Bar = 200 μm. b Tau (AT8) immunostaining of the corresponding area of a nearby section to a. Tau pathology is more prominent in the senile plaque-rich/cap-CAA-poor left half than in the senile plaque-poor/cap-CAA-rich right half. b, c are the same magnification as a. c Cresyl violet staining of the corresponding area of a nearby section to a. Dark vascular labeling is cyclooxygenase-2 immunostaining, which is irrelevant to the present study
The majority of capCAA show the typical morphology of globular deposits or linear thin layers associated with the capillary wall (Fig. 4a). In some capCAA, however, Aβ deposits appear to extend into the neuropil, forming senile plaque-like deposits. Such deposits are often associated with tau positive processes (Fig. 4b). This contrasts with the typical capCAA, which lacks heavy tau accumulation within the Aβ deposits as well as in the surrounding neuropil (Fig. 4a).
Double immunostaining for Aβ (E50: brown) and tau (AT8: purple). a capCAA without extravascular Aβ deposits. Bar = 20 μm. b capCAA with extravascular Aβ deposits. Tau accumulation is seen within the Aβ deposits and in the surrounding neuropil. Note that the diameter of the vessel within the Aβ deposits is about 10 μm or less [37]. The same magnification as a
To reveal the relationship between capCAA, non-capCAA, senile plaques and such tau pathology as NFT and NT, the degree of each pathology was scored from—(none), 1+ (few but present) to 5+ (most frequent) in double-immunostaining for Aβ and tau. Since CAA-associated plaque-like Aβ deposits are difficult to distinguish from senile plaques at low power magnification, and such deposits are similar to senile plaques with respect to the association of tau pathology, they were evaluated together as amyloid plaques. Representative visual fields of 1+, 3+ and 5+ of capCAA, non-capCAA, amyloid plaques and NT are shown in Fig. 5. In addition to NT, we also scored the occurrence of NFT by the number of NFT in a visual field. In each tissue section, we evaluated a visual field where capCAA was most frequent, together with a nearby field in the same cortical layer in which capCAA was rare (Table 2). We then examined whether the severity of non-capCAA, amyloid plaques, NFT and NT was different between the cap-CAA-frequent and cap-CAA-rare small areas by the Wilcoxon’s signed-rank test. The cap-CAA-frequent small areas showed significantly (P = 0.0039) lower score than the cap-CAA-rare small areas for amyloid plaques, NFT and NT. The score for non-capCAA did not differ significantly between the cap-CAA-frequent and cap-CAA-rare small areas (P = 0.8750). These results confirmed the qualitative analyses indicating that frequent capCAA and frequent amyloid plaques do not occur simultaneously in the same small area and that, in small areas with frequent capCAA, such tau pathology as NT and NFT is less intense than in adjacent small areas with frequent amyloid plaques.
Discussion
Amyloid β-protein is produced continuously in the brain. Under physiological conditions, production of Aβ is balanced by removal of Aβ from the brain. AD may be a condition where the balance is disturbed by pathological changes in Aβ metabolism such as overproduction, impaired degradation or efflux, and a shift in isoform or other changes in the nature of Aβ. Following release to the extracellular milieu, Aβ is removed from the brain by multiple pathways. Figure 6 summarizes the current understanding of the pathways for Aβ removal. Aβ is degraded by some proteases, of which neprilysin is the best studied [26, 41]. In culture, astrocytes and microglia take up Aβ dissolved in the culture medium [39]. An excess Aβ load causes intracellular accumulation of Aβ in these cells, a process that may model granular Aβ accumulation in quiescent glial cells observed in postmortem brain tissues [1, 3]. Aβ once deposited as insoluble aggregates can also be removed by activated microglia, a process that is associated with neuroinflammation [4]. The abundant Aβ deposits found in postmortem brain tissues indicate that the removal is at best partial in AD by itself. Further activation of microglia, which may occur in association with such complications as ischemia, can result in successful removal of the Aβ deposits [5].
Other physiological pathways involve Aβ efflux by the perivascular interstitial fluid drainage channel [48] to the cerebrospinal fluid and by the blood-brain-barrier (BBB) transport to the blood [51]. Aβ overload to these pathways may cause non-capCAA and capCAA, respectively. Such a notion is supported by transgenic mouse models of AD, in which mutant human APP is over-expressed in neurons [18, 19, 40]. In these models, CAA occurs in addition to parenchymal Aβ deposits as a consequence of Aβ overproduction by neurons.
In the present study, we have confirmed that Aβ40 predominates in non-capCAA and Aβ42 in capCAA. The propensity of each Aβ isoform to aggregate seems to explain the difference between non-capCAA and capCAA. To be excreted through the perivascular channel, Aβ needs to remain soluble for a while so that it can move for some distance in the neuropil to reach the arteries or arterioles. Capillaries, on the other hand, are so frequent that the less soluble Aβ42 may have access to BBB transport by capillary endothelial cells. The Aβ42/Aβ40 ratio is, therefore, expected to be much smaller in the perivascular channel than in the capillary route. The occurrence of Aβ42-positive capCAA is likely to indicate excess Aβ42 in the surrounding neuropil.
The relationship between CAA and senile plaques has been a matter of long-lasting debate. Some authors found no or even inverse association of CAA with senile plaques [44], while others found significant positive relationship [8, 43]. In these studies, however, no distinction was made between capCAA and non-capCAA. Recently, Attems and Jellinger [6] and Attems et al. [7] reported a better correlation between capCAA and Aβ42 positive senile plaques. In these studies, however, comparison was made between cases by evaluating data obtained from large cortical regions. In the present study, we focused on the difference in the distribution of capCAA and amyloid plaques between relatively small areas within the same cortical region of each case. We found that, in a given cortical region, capCAA-frequent small areas tended to be relatively poor in amyloid plaques and vice versa. CapCAA-prone small areas and plaque-prone small areas were often located close to each other. Our observation, therefore, is not inconsistent with the notion that capCAA and plaques correlate if one considers difference between large cortical regions or between cases and that frequent capCAA and plaques are indicative of parenchymal Aβ42 overload [6, 7]. Factors that determine the outcome of Aβ42 overload, i.e., capCAA- or senile plaque-formation, remains to be determined.
In AD, abnormally phosphorylated tau accumulates as NFT and NT. The occurrence of NFT and NT is not specific to AD but is considered to be more directly related to neuronal degeneration than is Aβ [25]. It has been an issue of long-lasting debate as to whether and how Aβ causes the formation of NFT/NT [11, 27]. A small number of NFT appear in the absence of Aβ deposits in the brain of non-demented aged subjects [10]. NFT/NT in normal aging, however, are far fewer than those in AD, indicating that pathophysiology of tau accumulation in such conditions is not identical to that in AD. Recent investigations have accumulated compelling evidence that Aβ induces the development of tau pathology in AD [22–24, 28]. Transgenic (Tg) mice expressing both mutant APP of AD and mutant tau of familial fromtotemporal dementia develop enhanced corticolimbic tau pathology compared to single tau-Tg mice [29]. Aβ injection into the brain of tau-Tg mice also enhances tau pathology [21]. A more recent study of APP/tau/presenilin-1 triple Tg mice has shown that Aβ removal by immunotherapy reduces tau pathology and that the Aβ deposits reemerge prior to that of tau pathology [34]. These observations all indicate that, in AD, Aβ induces tau-pathology in the presence of a yet-undetermined, possibly brain aging-related, factor that enhances a propensity of tau accumulation. An issue, therefore, seems to be which form of Aβ induces tau pathology.
At present, a significant number of investigators speculate that a soluble form of Aβ such as an Aβ oligomer may play an important role for neuronal degeneration [9, 17, 47]. A number of previous studies showed a positive relationship between tau pathology and CAA [8, 43, 49] or capCAA [6, 7], indicating that Aβ42 overload may cause both capCAA and senile plaques and, then, accelerate tau pathology. These studies assessed each cortical region as a whole and analyzed the difference between cases. In the present study, we sought for tissues containing both capCAA-frequent/plaque-rare small areas and capCAA-rare/plaque-frequent small areas near each other within the same cortical layer and region. These small areas appear to share similar cytoarchitectonics and fiber connections. As mentioned above, these small areas are also supposed to be similar with respect to Aβ42 overload, but they differ in the forms of resultant Aβ42 accumulation. The difference in the degree of tau pathology between these small areas indicates that parenchymal Aβ42 deposits promote abnormal accumulation of tau whereas Aβ42 positive capCAA does not. In the capCAA-frequent/plaque-rare small areas, excess Aβ in the interstitial fluid seems to move to capillaries without deposition in the parenchyma. We therefore speculate that, with a similar level of Aβ42 overload, movement of Aβ42 to the capillaries causes relatively less tau pathology than deposition to the parenchyma.
In summary, we have shown that Aβ42-predominant capCAA occurs in clusters in a small number of cases and that, in such cases, frequent capCAA is negatively associated with parenchymal Aβ42 plaques, NFT and NT within a given cortical region. Aβ42 may exert its neurotoxic effect that is related to NFT/NT formation in the form of insoluble, parenchymal deposits.
Reference
Akiyama H, Schwab C, Kondo H, Mori H, Kametani F, Ikeda K, McGeer PL (1996) Granules in glial cells of patients with Alzheimer’s disease are immunopositive for C-terminal sequences of beta-amyloid protein. Neurosci Lett 206:169–172
Akiyama H, Mori H, Sahara N, Kondo H, Ikeda K, Nishimura T, Oda T, McGeer PL (1997) Variable deposition of amyloid beta-protein (A beta) with the carboxy-terminus that ends at residue valine40 (A beta 40) in the cerebral cortex of patients with Alzheimer’s disease: a double-labeling immunohistochemical study with antibodies specific for A beta 40 and the A beta that ends at residues alanine42/threonine43 (A beta 42). Neurochem Res 22:1499–1506
Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL (1999) Occurrence of the diffuse amyloid beta-protein (Abeta) deposits with numerous Abeta-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia 25:324–331
Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K (2000) Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord 14 (Suppl 1):S47–S53
Akiyama H, McGeer PL (2004) Specificity of mechanisms for plaque removal after A beta immunotherapy for Alzheimer disease. Nat Med 10:117–118
Attems J, Jellinger KA (2004) Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology—a pilot study. Acta Neuropathol 107:83–90
Attems J, Lintner F, Jellinger KA (2004) Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol 107:283–291
Attems J, Jellinger KA, Lintner F (2005) Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol 110:222–231
Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H (2005) Globular amyloid beta-peptide oligomer—a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem 95:834–847
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Busciglio J, Lorenzo A, Yeh J, Yankner BA (1995) beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14:879–888
Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M (1999) Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci USA 96:14088–14093
Christie R, Yamada M, Moskowitz M, Hyman B (2001) Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol 158:1065–1071
Christoforidis M, Schober R, Krohn K (2005) Genetic-morphologic association study: association between the low density lipoprotein-receptor related protein (LRP) and cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 31:11–19
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci 23:8844–8853
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Shoepp DD, PaulSM, Mennerick S, Holtzman DM (2005) Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48:913–922
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–32053
Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV (2004) LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43:333–344
DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM (2002) Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295:2264–2267
Galuske RA, Drach LM, Nichtweiss M, Marquardt G, Franz K, Bohl J, Schlote W (2004) Colocalization of different types of amyloid in the walls of cerebral blood vessels of patients suffering from cerebral amyloid angiopathy and spontaneous intracranial hemorrhage: a report of 5 cases. Clin Neuropathol 23:113–119
Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Abeta 42 fibrils. Science 293:1491–1495
Gotz J, Schild A, Hoerndli F, Pennanen L (2004) Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci 22:453–465
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hardy J (2003) The relationship between amyloid and tau. J Mol Neurosci 20:203–206
Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ, Grundke-Iqbal I (1998) Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl 53:169–180
Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552
Katsuno T, Morishima-Kawashima M, Saito Y, Yamanouchi H, Ishiura S, Murayama S, Ihara Y (2005) Independent accumulations of tau and amyloid beta-protein in the human entorhinal cortex. Neurology 64:687–692
LaFerla FM, Oddo S (2005) Alzheimer’s disease: Abeta, tau and synaptic sysfunction. Trends Mol Med 11:170–176
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D Eckman C, Hardy J, Hutton M, McGowan E (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47:1113–1124
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L, participating CERAD neuropathologists (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mori H, Takio K, Ogawara M, Selkoe DJ (1992) Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J Biol Chem 267:17082–17086
Mori H, Ishii K, Tomiyama T, Furiya Y, Sahara N, Asano S, Endo N, Shirasawa T, Takio K (1994) Racemization: its biological significance on neuropathogenesis of Alzheimer’s disease. Tohoku J Exp Med 174:251–262
Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM (2004) Aβ immunotherapy leads to cliearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43:321–332
Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ (1995) Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol 52:702–708
Olichney JM, Ellis RJ, Katzman R, Sabbagh MN, Hansen L (1997) Types of cerebrovascular lesions associated with severe cerebral amyloid angiopathy in Alzheimer’s disease. Ann N Y Acad Sci 826:493–497
Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO (2003) Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 29:106–117
Selkoe DJ (2001) Alzheimer’s disease; genes, proteins, and therapy. Physiol Rev 81:741–766
Shaffer LM, Dority MD, Gupta-Bansal R, Frederickson RCA, Younkin SG, Brunden KR (1995) Amyloid β protein (Aβ) removal by neuroglial cells in culture. Neurobiol Aging 16:737–745
Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV (2000) Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489–1499
Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, Kiryu-Seo S, Kiyama H, Iwata H, Tomita T, Iwatsubo T, Saido TC (2001) Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem 276:21895–21901
Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Tredici KD, Braak H (2002) Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–293
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Tian J, Shi J, Bailey K, Mann DM (2003) Negative association between amyloid plaques and cerebral amyloid angiopathy in Alzheimer’s disease. Neurosci Lett 352:137–140
Tian J, Shi J, Bailey K, Lendon CL, Pickering-Brown SM, Mann DM (2004) Association between apolipoprotein E e4 allele and arteriosclerosis, cerebral amyloid angiopathy, and cerebral white matter damage in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75:696–699
Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–649
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE (1998) Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 153:725–733
Williams S, Chalmers K, Wilcock GK, Love S (2005) Relationship of neurofibrillary pathology to cerebral amyloid angiopathy in Alzheimer’s disease. Neuropathol Appl Neurobiol 31:414–421
Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L (2001) TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med 7:612–618
Zlokovic BV (2004) Clearing amyloid through the blood-brain barrier. J Neurochem 89:807–811
Acknowledgement
We thank Prof. Hiroshi Mori for providing antibodies to Aβ carboxyl terminals. This research was supported by grant in aid for scientific research from the ministry of education, culture, sports, science and technology (14570957).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshima, K., Akiyama, H., Tsuchiya, K. et al. Relative paucity of tau accumulation in the small areas with abundant Aβ42-positive capillary amyloid angiopathy within a given cortical region in the brain of patients with Alzheimer pathology. Acta Neuropathol 111, 510–518 (2006). https://doi.org/10.1007/s00401-006-0070-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0070-z