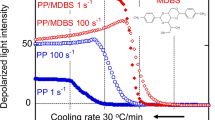
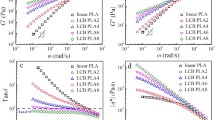
Abstract
In this paper, the effect of shear on the flow-induced crystallization (FIC) of several polypropylenes of various macrostructures was studied using rheometry combined with polarized microscopy. Generally, an increase in strain and strain rate or decrease of temperature is found to decrease the thermodynamic barrier for crystal formation and thus enhancing crystallization kinetics at temperatures between the melting and crystallization points. Secondly, popular models based on suspension theory which are used to relate the degree of crystallinity to normalized rheological functions (such as viscosity) are validated experimentally. For this purpose, the space filling of crystals in the polarized micrographs determined from image processing was plotted as a function of normalized viscosity under various shear rates. It is found that the constant(s) of various suspension models should be dependent on the flow parameters in order for the suspension models to describe the effect of shear on FIC, particularly at higher shear rates.

















Similar content being viewed by others
References
Acierno S, Grizzuti N (2003) Measurements of the rheological behavior of a crystallizing polymer by an inverse quenching technique. J Rheol 47:563
Acierno S, Palomba B, Winter H H, Grizzuti N (2003) Effect of molecular weight on the flow-induced crystallization of isotactic poly (1-butene). Rheol Acta 42(3):243–250
Ansari M, Hatzikiriakos S G, Sukhadia A M, Rohlfing D C (2011) Rheology of Ziegler–Natta and metallocene high-density polyethylenes: broad molecular weight distribution effects. Rheol Acta 50(1):17–27
Baert J, Puyvelde P V, Langouche F (2006) Flow-induced crystallization of PB-1: from the low shear rate region up to processing rates. Macromolecules 39(26): 9215–9222
Baert J, Van Puyvelde P (2006) Effect of molecular and processing parameters on the flow-induced crystallization of poly-1-butene. Part 1: kinetics and morphology. Polymer 47(16):5871–5879
Ball R C, Richmond P (1980) Dynamics of colloidal dispersions. Phys Chem Liq 9(2):99–116
Bourgin P, Zinet M (2010) Thermally and flow induced crystallization of polymers at low shear rate. J Non-Newton Fluid Mech 165(5):227–237
Boutahar K, Carrot C, Guillet J (1998) Crystallization of polyolefins from rheological measurements relation between the transformed fraction and the dynamic moduli. Macromolecules 31(6):1921–1929
Chellamuthu M, Arora D, Winter H H, Rothstein J P (2011) Extensional flow-induced crystallization of isotactic poly-1-butene using a filament stretching rheometer. J. Rheol 55:901
Coppola S, Balzano L, Gioffredi E, Maffettone P L, Grizzuti N (2004) Effects of the degree of undercooling on flow induced crystallization in polymer melts. Polymer 45(10):3249–3256
Dai S C, Qi F, Tanner R I (2006) Strain and strain-rate formulation for flow-induced crystallization. Polymer Eng Sci 46(5):659–669
Derakhshandeh M, Hatzikiriakos S G (2012) Flow-induced crystallization of high-density polyethylene: the effects of shear and uniaxial extension. Rheol Acta 51(4):315–327
Devaux N, Monasse B, Haudin J M, Moldenaers P, Vermant J (2004) Rheooptical study of the early stages of flow enhanced crystallization in isotactic polypropylene. Rheol Acta 43(3):210–222
D’Haese M, Goderis B, Van Puyvelde P (2011) The influence of calcium-stearate-coated calcium carbonate and talc on the quiescent and flow-induced crystallization of isotactic poly (propylene). Macromol Mater Eng 296(7):603–616
D’Haese M, Van Puyvelde P, Langouche F (2010) Effect of particles on the flow-induced crystallization of polypropylene at processing speeds. Macromolecules 43(6):2933–2941
Doufas A K, Dairanieh I S, McHugh A J (1999) A continuum model for flow-induced crystallization of polymer melts. J Rheol 43:85–109
Doufas A K, McHugh A J, Miller C (2000) Simulation of melt spinning including flow-induced crystallization: part I. Model development and predictions. J Non-Newton Fluid Mech 92(1):27–66
Doufas A K, McHugh A J (2001) Simulation of film blowing including flow-induced crystallization. J Rheol 45:1085
Doufas A K (2013) A microstructural flow-induced crystallization model for film blowing: validation with experimental data. Rheol Acta 53:269–293
Duplay C, Monasse B, Haudin J M, Costa J L (2000) Shear-induced crystallization of polypropylene: influence of molecular weight. J Mater Sci 35(24):6093–6103
Eder G, Janeschitz-Kriegl H, Liedauer S (1990) Crystallization processes in quiescent and moving polymer melts under heat transfer conditions. Progr Polymer Sci 15(4):629–714
Ferry J D (1980) Viscoelastic properties of polymers, vol 3. Wiley, New York, p 641
Frankel N A, Acrivos A (1967) On the viscosity of a concentrated suspension of solid spheres. Chem Eng Sci 22(6):847–853
Eder G, Janeschitz-Kriegl H, Meijer HEH (eds) (1998) Materials science and technology, vol 18, chap 5. Wiley, Weinheim
Gelfer Y, Winter H H (1999) Effect of branch distribution on rheology of LLDPE during early stages of crystallization. Macromolecules 32(26):8974–8981
Godara A, Raabe D, Van Puyvelde P, Moldenaers P (2006) Influence of flow on the global crystallization kinetics of iso-tactic polypropylene. Polymer Test 25(4):460–469
Graham A L (1981) On the viscosity of suspensions of solid spheres. Appl Sci Res 37(3–4):275–286
Hadinata C, Gabriel C, Ruellman M, Laun H M (2005) Comparison of shear-induced crystallization behavior of PB-1 samples with different molecular weight distribution. J Rheol 49(1):327–349
Hadinata C, Gabriel C, Ruellmann M, Kao N, Laun H M (2006) Shear-induced crystallization of PB-1 up to processing-relevant shear rates. Rheola Acta 45(5):539–546
Hadinata C, Boos D, Gabriel C, Wassner E, Rüllmann M, Kao N, Laun H M (2007) Elongation-induced crystallization of a high molecular weight isotactic polybutene-1 melt compared to shear-induced crystallization. J Rheol 51(2):195
Han C D, Kwack T H (1983) Rheology-processing-property relationships in tubular blown film extrusion. I. High-pressure low-density polyethylene. J Appl Polymer Sci 28(11):3399–3418
Huo H, Jiang S, An L, Feng J (2004) Influence of shear on crystallization behavior of the β phase in isotactic polypropylene with β-nucleating agent. Macromolecules 37(7):2478–2483
Jay F, Haudin J M, Monasse B (1999) Shear-induced crystallization of polypropylenes: effect of molecular weight. J Mater Sci 34(9):2089–2102
Janeschitz-Kriegl H (2003) How to understand nucleation in crystallizing polymer melts under real processing conditions. Colloid Polymer Sci 281(12):1157–1171
Janeschitz-Kriegl H, Ratajski E (2005) Kinetics of polymer crystallization under processing conditions: transformation of dormant nuclei by the action of flow. Polymer 46(11):3856–3870
Janeschitz-Kriegl H, Ratajski E, Stadlbauer M (2003) Flow as an effective promotor of nucleation in polymer melts: a quantitative evaluation. Rheologica Acta 42(4):355–364
Kataoka T, Kitano T, Sasahara M, Nishijima K (1978) Viscosity of particle filled polymer melts. Rheol Acta 17(2):149–155
Kim K H, Isayev A I, Kwon K (2005) Flow-induced crystallization in the injection molding of polymers: a thermodynamic approach. J Appl Polymer Sci 95(3):502–523
Kitano T, Kataoka T, Shirota T (1981) An empirical equation of the relative viscosity of polymer melts filled with various inorganic fillers. Rheol Acta 20(2):207–209
Kitoko V, Keentok M, Tanner R I (2003) Study of shear and elongational flow of solidifying polypropylene melt for low deformation rates. Korea Aust Rheol J 15:63–73
Koscher E, Fulchiron R (2002) Influence of shear on polypropylene crystallization: morphology development and kinetics. Polymer 43(25):6931–6942
Kornfield J A, Kumaraswamy G, Issaian A M (2002) Recent advances in understanding flow effects on polymer crystallization. Ind Eng Chem Res 41(25):6383–6392
Kuijk E W, Tas P P, Neuteboom P (1999) A rheological model for the prediction of polyethylene blown film properties. J Reinforc Plast Compos 18(6):508–517
Kumaraswamy G, Issaian A M, Kornfield J A (1999) Shear-enhanced crystallization in isotactic polypropylene. 1. Correspondence between in situ rheo-optics and ex situ structure determination. Macromolecules 32(22):7537–7547
Kwack T H, Han C D (1983) Rheology-processing-property relationships in tubular blown film extrusion. II. Low-pressure low-density polyethylene. J Appl Polymer Sci 28(11):3419–3433
Lamberti G, Peters G W M, Titomanlio G (2007) Crystallinity and linear rheological properties of polymers. Int Polymer Process 22(3):303–310
Macosko C W (1994) Rheology: principles, measurements, and applications, 1st edn. Wiley, New York
McHugh AJ, Edwards BJ (1995) Flow-induced structure formation in polymer solutions. In: LJ Jorgensen, K Sondergaard (eds) Rheo-Physics of Multiphase Polymeric Systems, vol 5. Technomic Publishing Company, Lancaster, pp 227–267
Mooney M (1951) The viscosity of a concentrated suspension of spherical particles. J Colloid Sci 6(2):162–170
Natta G, Pino P, Corradini P, Danusso F, Mantica E, Mazzanti G, Moraglio G (1955) Crystalline high polymers of α-olefins. J Am Chem Soc 77(6):1708–1710
Pantani R, Speranza V, Titomanlio G (2001) Relevance of crystallisation kinetics in the simulation of the injection molding process. Int Polymer Process 16(1):61–71
Paradkar R P, Patel R M, Knickerbocker E, Doufas A K (2008) Raman spectroscopy for spinline crystallinity measurements. I. Experimental studies. J Appl Polymer Sci 109(5):3413–3420
Patel R M, Doufas A K, Paradkar R P (2008) Raman spectroscopy for spinline crystallinity measurements. II. Validation of fundamental fiber-spinning models. J Appl Polymer Sci 109(5):3398–3412
Scelsi L, Mackley M R (2008) Rheo-optic flow-induced crystallisation of polypropylene and polyethylene within confined entry–exit flow geometries. Rheol Acta 47(8):895–908
Stadlbauer M, Janeschitz-Kriegl H, Eder G, Ratajski E (2004) New extensional rheometer for creep flow at high tensile stress. Part II. Flow induced nucleation for the crystallization of iPP. J Rheol 48:631–641
Sun T, Brant P, Chance R R, Graessley W W (2001) Effect of short chain branching on the coil dimensions of polyolefins in dilute solution. Macromolecules 34:6812–6820
Swartjes FHM, Peters GW, Rastogi S, Meijer HE (2001) Stress induced crystallization in elongational flow. Technische Universiteit Eindhoven
Tanner R I, Qi F (2005) A comparison of some models for describing polymer crystallization at low deformation rates. J Non-Newton Fluid Mech 127(2):131–141
Tanner R I, Qi F (2009) Stretching, shearing and solidification. Chem Eng Sci 64(22):4576–4579
Tiang J S, Dealy J M (2012) Shear induced crystallization of isotactic polypropylene studied by simultaneous light intensity and rheological measurements. Polymer Eng Sci 52(4):835–848
Titomanlio G, Speranza V, Brucato V (1997) On the simulation of thermoplastic injection moulding process. 2. Relevance of interaction between flow and crystallization. Int Polymer Process 12(1):45–53
Wang J, Dou Q (2008) Crystallization behaviors and optical properties of isotactic polypropylene: comparative study of a trisamide and a rosin-type nucleating agent. Colloid Polymer Sci 286(6-7):699–705
White E E B, Winter H H, Rothstein J P (2012) Extensional-flow-induced crystallization of isotactic polypropylene. Rheol Acta 51(4):303–314
Wunderlich B (1976) Macromolecular physics, crystal nucleation, growth, annealing, V2. Academic, New York
Acknowledgments
Financial assistance from the Natural Sciences and Engineering Research Council (NSERC) of Canada, the scholarship program of the University of British Columbia (4YF), and the ExxonMobil Chemical are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
The coupled energy equations for the system shown in Fig. 18 where the polymer in the space between two quartz plates is crystallized are developed. This is necessary to calculate the increase of temperature in the optical microscopy results due to the heat of crystallization which is accumulated in the sample. This has an effect on the degree of crystallinity which needs to be quantified in order to explain the differences between the POM and DSC results with reference to Fig. 16a.
Crystal formation releases heat within the polymer at a rate of
where \(\Delta H_{f\left ({100\% \;\text {crystalline}\;\text {polymer}} \right )} \) is the heat of melting if the polymer was 100 % crystalline, ρ is the density of crystals, and \(\dot {\emptyset }\) is the rate of volume fraction increase of crystals. The heat transfer equations for the polymer and the quartz plate located on top of the polymer (Fig. 18) are as follows:
The equations are solved using POLYFLOW with the following boundary conditions. A convection boundary condition is imposed on the plate in contact with nitrogen gas which is purged into the oven chamber of the rheometer as the heating/cooling gas. The heat transfer coefficient used in these calculations was h = 100 W/(m 2⋅K). The quartz plate underneath the polymer imposes an adiabatic boundary condition at the lower side of the polymer. Continuity of T and heat rate at the polymer/quartz interface completes the required BC.s.
After solving the system of Eq. 8, the temperature elevation within the polymer sample can be obtained as a function of T. The temperature rise as a function of time at r = 0.75R and z = 0.25 mm is shown in Fig. 19. The temperature rise in the polymer matrix decreased as the temperature is increased. At higher temperatures, the kinetics of crystallization slows down, and thus less heat is released per unit of time. This essentially explains the larger differences between OM and DSC results depicted in Fig. 16a at the lower temperatures.
The temperature rise at r/ R = 0.75 and z/ h = 0.25 in a polymer sample that crystallizes at different temperatures. The temperature increases of 0.2 to 1.9 °C at the crystallization temperatures of 131.4 to 121.7 °C, respectively, explain adequately the differences in Fig. 16a
Appendix B
The Avrami equation is often used to predict the isothermal crystallization behavior under quiescent condition. This equation can be written as follows:
where X is the degree of crystallinity, X f is the total crystallinity at the end of primary crystallization process, n is the Avrami index with a value of 2 for the resin studied, and k is the Avrami rate parameter. The Avrami parameter k contains the temperature dependence of the nucleation and crystal growth processes, while the exponent n depends on the geometry and dimensionality of the growth as well as on the nature of the nucleation process. The Avrami equation best predicts the behavior in primary growth region of crystals which corresponds to a relative crystallinity in between 30 and 70 %. The Avrami index determined experimentally varied in the range of 1.9–2.5, and therefore, the theoretical value of 2 (1.9–2.7) was chosen for the investigated temperatures (120–135 °C). The Arrhenius Eq. 10 is used in this study to represent the dependency of the Avrami rate parameter on temperature. The Arrhenius equation is then used in combination with the Avrami equation to predict the behavior of the sample in the microscopy setup.
The value of the Avrami rate parameter obtained using the temperature history in Fig. 19 along with the Arrhenius equation is used in the Avrami equation (Fig. 20). The prediction of the Avrami model is shown in Fig. 16b and compared with the experimental data from the microscopy setup. The agreement was found to be excellent which shows that the difference in Fig. 16a is related to different thermal histories during crystallization.
Rights and permissions
About this article
Cite this article
Derakhshandeh, M., Doufas, A.K. & Hatzikiriakos, S.G. Quiescent and shear-induced crystallization of polyprophylenes. Rheol Acta 53, 519–535 (2014). https://doi.org/10.1007/s00397-014-0775-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-014-0775-1