Abstract
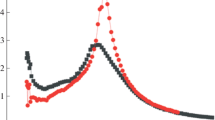
As we determined visually by the temperature cloud point method, the coexistence phase curve of methylcellulose in aqueous solution belongs to the LCST (low critical solution temperature) type. Rheological dynamic measurements reveal the existence of three gel domains. The gel (I) localized in the homogeneous phase at low concentration and low temperature, is a very weak gel, where the cross-links are attributed to pairwise hydrophobic interactions between the most hydrophobic zones of the backbone: the trimethyl blocks. The second gel (II) was revealed in the high concentration regime and below the LCST, it may be attributed to the formation of crystallites which play the role of cross-linking points. The third gel was concomitant to the micro-phase separation. In these turbid gels, syneresis develops slowly with time: the higher the temperature and the lower the concentration, the faster the syneresis. Near the three sol-gel transitions, a power law frequency dependence of the loss and storage moduli was observed and the viscoelastic exponent Δ(G′ G″ ω Δ) was found to be 0.76 and 0.8 and to reach 1 at high concentration.
Similar content being viewed by others
References
Heymann E (1935) Trans Farad Soc 31:846–864
Sarkar N (1979) Appl Polym Sci 24: 1073–1087
Grover JA (1986) In: Glicksman M (ed) Food Hydrocolloids, Vol III, CRC Press, Boca Raton, pp 121–154
Greminger GK, Savage AB (1959) In: Whistler RL, BeMiller JN (eds) Industrial Gums-Polysaccharides and their derivatives. Academic Press, New York, pp 565–596
Arisz PW, Kauw HJJ, Boon JJ (1995) Carbohydr Res 271:1–14
Doelker E (1990) Stud Polym Sci 8: 125–145
Koda S, Hori T, Nomura H, Kawaizumi F (1991) Polymer 32:2806–2810
Rees DA (1972) Chem Ind London 630
Sarkar N (1995) Carbohydr Polym 26:195–203
Khomutov LI, Ryskina II, Panina NI, Dubina LG, Timofeeva GH (1993) Polym Sci 35:320–323
Kawanishi K, Komatsu M, Inoue T (1987) Polymer 28:980–984
Palma-Vittorelli MB (1989) Int J Quantum Chem 35:113–124
Matsuo M, Kawase M, Sugiura Y, Takematsu S, Hara C (1993) Macro-molecules 26:4461–4471
Vigouret M, Rinaudo M, Desbrières J (1996) J Chim Phys 93:858–869
Haque A, Morris ER (1993) Carbohydr Polym 22:161–173
Flory PJ (1978) Principles of Polymer Chemistry. Cornell University Press, Ithaca and London
Hirrien M, Desbrières J, Rinaudo M (1996) Carbohydr Polym, in press
Devreux F, Boilot JP, Chaput F, Malier L, Axelos MAV (1993) Phys Rev E 47:2689–2694
Axelos MAV, Kolb M (1990) Phys Rev Lett 64:1457–1460
Durand D, Delsanti M, Adam M, Luck JM (1987) Europhys Lett 3:297–301
Stauffer D (1985) Introduction to Percolation Theory. Taylor & Francis, London and Philadelphia
Lárez-VC, Crescenzi V, Ciferri A (1995) Macromolecules 28:5280–5284
Cabane B, Lindell K, Engström S, Lindamn B (1996) Macromolecules 29: 3188–3197
Johansson HO, Karlström G, Tjerneld F (1993) Macromolecules 26:34478–4483
Takahashi SI, Fujimoto T, Miyamoto T, Inagaki H (1987) J Polym Sci: Part A Polym Chem 25:987–994
Miyamoto T, Long M, Donkai N (1995) Macromol Symp 99:141–147
Kato T, Yokoyama M, Takahashi A (1978) Colloid & Polym Sci 256:15–21
Nyström B, Walderhaug H, Hansen FK (1995) Langmuir 11:750–757
Michon C, Cuvelier G, Launay B (1993) Rheol Acta 32:94–103
San Biagio PL, Bulone D, Emanuele A, Madonia F, Di Stefano L, Giacomazza D, Trapanese M, Palma-Vittorelli MB, Palma MU (1990) Makromol Chem Macromol Symp 40:33–44
Luan CH, Parker TM, Gowda DC, Urry DW (1992) Biopolymers 32:1251–1261
Lewis KE, Robinson CP (1970) J Colloid Interface Sci 32:539–546
Chevillard C, Axelos MAV, to be published
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chevillard, C., Axelos, M.A.V. Phase separation of aqueous solution of methylcellulose. Colloid Polym Sci 275, 537–545 (1997). https://doi.org/10.1007/s003960050116
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s003960050116