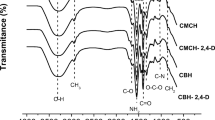
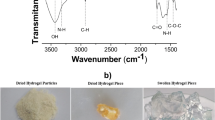
Abstract
The aim of the present study was to evaluate a chitosan-based hydrogel and chitosan/magnetite-based composite hydrogel as potential matrices for water treatment through sorption experiments involving metals and dyes. Cadmium and methylene blue were used as model pollutants. The best isotherm fits were found using three-parameter isotherms, indicating both the formation of a monolayer and multi-site interactions in the hydrogel networks due to the diffusion of the solutes and macromolecular relaxation of the polymer chains. Maximum methylene blue sorption capacities of the chitosan-based hydrogel and chitosan/magnetite-based composite hydrogel were 23.389 and 23.478 mg g−1, respectively. These values were respectively 90.038 and 80.383 mg g−1 for cadmium sorption. The best kinetic fit for the interaction of methylene blue and cadmium to the chitosan-based hydrogel was the nonlinear pseudo-second-order kinetic model, indicating that a chemical reaction controls the adsorption rate between the hydrogel and pollutant. The opposite was found for interaction with the chitosan/magnetite-based composite hydrogel, suggesting that the active-site occupation rate is proportional to the number of non-occupied active sites. The thermodynamic results revealed that the sorption processes were favorable and endothermic, with the possible occurrence of physical interactions. However, hydrogel swelling can alter the sorption isotherm, kinetics, and thermodynamics. The interaction of methylene blue and cadmium with both hydrogels was confirmed by Fourier-transform infrared spectroscopy and thermogravimetric analyses.









Similar content being viewed by others
References
Jiang C, Wang X, Wang G, Hao C, Li X, Li T (2019) Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos Part B Eng 169:45–54. https://doi.org/10.1016/j.compositesb.2019.03.082
Zhao Z, Chen H, Zhang H, Ma L, Wang Z (2017) Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens Bioelectron 91:306–312. https://doi.org/10.1016/j.bios.2016.12.047
Shang Z, Zhang LW, Zhao X, Liu S, Li D (2019) Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercapto-modified coal gangue. J Environ Manag 231:391–396. https://doi.org/10.1016/j.jenvman.2018.10.072
Shakir SK, Azizullah A, Murad W et al (2017) Toxic metal pollution in Pakistan and its possible risks to public health. Rev Environ Contam Toxicol. https://doi.org/10.1007/398_2016_9
Mor S, Chhavi MK, Sushil KK, Ravindra K (2018) Assessment of hydrothermally modified fly ash for the treatment of methylene blue dye in the textile industry wastewater. Environ Dev Sustain 20:625–639. https://doi.org/10.1007/s10668-016-9902-8
Dong C, Lu J, Qiu B, Shen B, Xing M, Zhang J (2018) Developing stretchable and graphene-oxide-based hydrogel for the removal of organic pollutants and metal ions. Appl Catal B Environ 222:146–156. https://doi.org/10.1016/j.apcatb.2017.10.011
Qi X, Wu L, Su T, Zhang J, Dong W (2018) Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surf B: Biointerfaces 170:364–372. https://doi.org/10.1016/j.colsurfb.2018.06.036
Kataria N, Garg VK (2018) Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere. 208:818–828. https://doi.org/10.1016/j.chemosphere.2018.06.022
Shalla AH, Yaseen Z, Bhat MA, Rangreez TA, Maswal M (2019) Recent review for removal of metal ions by hydrogels. Sep Sci Technol 54:89–100
Agnihotri S, Singhal R (2018) Synthesis and characterization of novel poly (acrylic acid/sodium alginate/sodium Humate) superabsorbent hydrogels. Part II: the effect of SH variation on Cu2+, Pb2+, Fe2+ metal ions, MB, CV dye adsorption study. J Polym Environ 26:383–395. https://doi.org/10.1007/s10924-017-0956-y
Soleimani K, Tehrani ADD, Adeli M (2018) Bioconjugated graphene oxide hydrogel as an effective adsorbent for cationic dyes removal. Ecotoxicol Environ Saf 147:34–42. https://doi.org/10.1016/j.ecoenv.2017.08.021
Kim YW, Kim JE, Jung Y, Sun JY (2019) Non-swellable, cytocompatible pHEMA-alginate hydrogels with high stiffness and toughness. Mater Sci Eng C 95:86–94. https://doi.org/10.1016/j.msec.2018.10.045
Vieira RM, Vilela PB, Becegato VA, Paulino AT (2018) Chitosan-based hydrogel and chitosan/acid-activated montmorillonite composite hydrogel for the adsorption and removal of Pb+2 and Ni+2 ions accommodated in aqueous solutions. J Environ Chem Eng 6:2713–2723. https://doi.org/10.1016/j.jece.2018.04.018
Paulino AT, Guilherme MR, de Almeida EAMS, Pereira AGB, Muniz EC, Tambourgi EB (2009) One-pot synthesis of a chitosan-based hydrogel as a potential device for magnetic biomaterial. J Magn Magn Mater 321:2636–2642. https://doi.org/10.1016/j.jmmm.2009.03.078
Paulino AT, Belfiore LA, Kubota LT et al (2011) Efficiency of hydrogels based on natural polysaccharides in the removal of Cd 2+ ions from aqueous solutions. Chem Eng J 168:68–76. https://doi.org/10.1016/j.cej.2010.12.037
Singh NB, Nagpal G, Agrawal S, Rachna (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Tambourgi EB (2011) Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem Eng J 168:68–76. https://doi.org/10.1016/j.cej.2010.12.037
Paulino AT, Campese GM, Favaro SL et al (2007) Water absorption profile of PAAm-co-PNIPAAm/chitosan hydrogel with sandwich-like morphology. E-Polymers
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Khare AR, Peppas NA (1995) Swelling/deswelling of anionic copolymer gels. Biomaterials. 16:559–567. https://doi.org/10.1016/0142-9612(95)91130-Q
Vilela PB, Dalalibera A, Duminelli EC, Becegato VA, Paulino AT (2019) Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ Sci Pollut Res 26:28481–28489. https://doi.org/10.1007/s11356-018-3208-3
Norouzi S, Heidari M, Alipour V, Rahmanian O, Fazlzadeh M, Mohammadi-moghadam F, Nourmoradi H, Goudarzi B, Dindarloo K (2018) Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour Technol 258:48–56. https://doi.org/10.1016/j.biortech.2018.02.106
Kast W (1985) Principles of adsorption and adsorption processes. Chem Eng Process Process Intensif 19:118. https://doi.org/10.1016/0255-2701(85)80013-1
Dalalibera A, Vilela PB, Vieira T, Becegato VA, Paulino AT (2020) Removal and selective separation of synthetic dyes from water using a polyacrylic acid-based hydrogel: characterization, isotherm, kinetic, and thermodynamic data. J Environ Chem Eng 8:104465. https://doi.org/10.1016/j.jece.2020.104465
Paulino AT, Pereira AGB, Fajardo AR, Erickson K, Kipper MJ, Muniz EC, Belfiore LA, Tambourgi EB (2012) Natural polymer-based magnetic hydrogels: potential vectors for remote-controlled drug release. Carbohydr Polym 90:1216–1225. https://doi.org/10.1016/j.carbpol.2012.06.051
Guilherme MR, Aouada FA, Fajardo AR, Martins AF, Paulino AT, MFT D, Rubira AF, Muniz EC (2015) Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Eur Polym J 72:365–385. https://doi.org/10.1016/j.eurpolymj.2015.04.017
Paulino AT, Guilherme MR, Mattoso LHC, Tambourgi EB (2010) Smart hydrogels based on modified gum arabic as a potential device for magnetic biomaterial. Macromol Chem Phys 211:1196–1205. https://doi.org/10.1002/macp.200900657
Zheng Y, Huang D, Wang A (2011) Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal Chim Acta 687:193–200. https://doi.org/10.1016/j.aca.2010.12.026
Vilela PB, Matias CA, Dalalibera A, Becegato VA, Paulino AT (2019) Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: equilibrium isotherm, kinetic and thermodynamic studies. J Environ Chem Eng 7:103327. https://doi.org/10.1016/j.jece.2019.103327
Falamarzpour P, Behzad T, Zamani A (2017) Preparation of nanocellulose reinforced chitosan films, cross-linked by adipic acid. Int J Mol Sci 18. https://doi.org/10.3390/ijms18020396
Milosavljević NB, Ristić MD, Perić-Grujić AA et al (2011) Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids Surfaces A Physicochem Eng Asp 388:59–69. https://doi.org/10.1016/j.colsurfa.2011.08.011
Tang J, Huang J, Zhou G, Liu S (2020) Versatile fabrication of ordered cellular structures double network composite hydrogel and application for cadmium removal. J Chem Thermodyn 141:105918. https://doi.org/10.1016/j.jct.2019.105918
Almodóvar J, Place LW, Gogolski J et al (2011) Layer-by-layer assembly of polysaccharide-based polyelectrolyte multilayers: a spectroscopic study of hydrophilicity, composition, and ion pairing. Biomacromolecules. 12:2755–2765. https://doi.org/10.1021/bm200519y
Pavlovic I, Barriga C, Hermosín MC et al (2005) Adsorption of acidic pesticides 2,4-D, clopyralid and picloram on calcined hydrotalcite. Appl Clay Sci 30:125–133. https://doi.org/10.1016/j.clay.2005.04.004
Kittur FS, Harish Prashanth KV, Udaya Sankar K, Tharanathan RN (2002) Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr Polym 49:185–193. https://doi.org/10.1016/S0144-8617(01)00320-4
Ostrowska-Czubenko J, Gierszewska-Druzyńska M (2009) Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr Polym 77:590–598. https://doi.org/10.1016/j.carbpol.2009.01.036
Lewandowska K (2009) Miscibility and thermal stability of poly(vinyl alcohol)/chitosan mixtures. Thermochim Acta 493:42–48. https://doi.org/10.1016/j.tca.2009.04.003
Simionato JI, Paulino AT, Garcia JC, Nozaki J (2006) Adsorption of aluminium from wastewater by chitin and chitosan produced from silkworm chrysalides. Polym Int 55:1243–1248. https://doi.org/10.1002/pi.2070
Pereira AGB, Martins AF, Paulino AT et al (2017) Recent advances in designing hydrogels from chitin and chitin-derivatives and their impact on environment and agriculture: a review. Rev Virtual Quim 9:370–386. https://doi.org/10.21577/1984-6835.20170021
Sun L, Hu S, Sun H, Guo H, Zhu H, Liu M, Sun H (2015) Malachite green adsorption onto Fe3O4@SiO2-NH2: isotherms, kinetic and process optimization. RSC Adv 5:11837–11844. https://doi.org/10.1039/c4ra13402h
Nightingale ER (1959) Phenomenological theory of ion solvation. Effective radii of hydrated ions. J Phys Chem 63:1381–1387. https://doi.org/10.1021/j150579a011
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Almeida VC, Tambourgi EB (2011) Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination. 275:187–196. https://doi.org/10.1016/j.desal.2011.02.056
Jiaqi Z, Yimin D, Danyang L, Shengyun W, Liling Z, Yi Z (2019) Synthesis of carboxyl-functionalized magnetic nanoparticle for the removal of methylene blue. Colloids Surfaces A Physicochem Eng Asp 572:58–66. https://doi.org/10.1016/j.colsurfa.2019.03.095
Kołodyńska D, Gęca M, Pylypchuk IV, Hubicki Z (2016) Development of new effective sorbents based on nanomagnetite. Nanoscale Res Lett 11:152. https://doi.org/10.1186/s11671-016-1371-3
Savić AB, Čokeša D, Lazarević S, Jokić B, Janaćković D, Petrović R, Živković LS (2016) Tailoring of magnetite powder properties for enhanced phosphate removal: effect of PEG addition in the synthesis process. Powder Technol 301:511–519. https://doi.org/10.1016/j.powtec.2016.06.028
Paulino AT, Santos LB, Nozaki J (2008) Removal of Pb2+, Cu2+, and Fe3+ from battery manufacture wastewater by chitosan produced from silkworm chrysalides as a low-cost adsorbent. React Funct Polym 68:634–642. https://doi.org/10.1016/j.reactfunctpolym.2007.10.028
Vilela PB, Dalalibera A, Duminelli EC, Becegato VA, Paulino AT (2018) Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ Sci Pollut Res 26:28481–28489. https://doi.org/10.1007/s11356-018-3208-3
Ncibi MC (2008) Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 153:207–212. https://doi.org/10.1016/j.jhazmat.2007.08.038
Sharififard H, Shahraki ZH, Rezvanpanah E, Rad SH (2018) A novel natural chitosan/activated carbon/iron bio-nanocomposite: Sonochemical synthesis, characterization, and application for cadmium removal in batch and continuous adsorption process. Bioresour Technol 270:562–569. https://doi.org/10.1016/j.biortech.2018.09.094
Martini BK, Daniel TG, Corazza MZ, De Carvalho AE (2018) Methyl orange and tartrazine yellow adsorption on activated carbon prepared from boiler residue: kinetics, isotherms, thermodynamics studies and material characterization. J Environ Chem Eng 6:6669–6679. https://doi.org/10.1016/j.jece.2018.10.013
Heidarinejad Z, Rahmanian O, Fazlzadeh M, Heidari M (2018) Enhancement of methylene blue adsorption onto activated carbon prepared from Date Press Cake by low frequency ultrasound. J Mol Liq 264:591–599. https://doi.org/10.1016/j.molliq.2018.05.100
Asouhidou DD, Triantafyllidis KS, Lazaridis NK, Matis KA, Kim SS, Pinnavaia TJ (2009) Sorption of reactive dyes from aqueous solutions by ordered hexagonal and disordered mesoporous carbons. Microporous Mesoporous Mater 117:257–267. https://doi.org/10.1016/j.micromeso.2008.06.034
Leechart P, Nakbanpote W, Thiravetyan P (2009) Application of “waste” wood-shaving bottom ash for adsorption of azo reactive dye. J Environ Manag 90:912–920. https://doi.org/10.1016/j.jenvman.2008.02.005
Djelad A, Mokhtar A, Khelifa A, Bengueddach A, Sassi M (2019) Alginate-whey an effective and green adsorbent for crystal violet removal: kinetic, thermodynamic and mechanism studies. Int J Biol Macromol 139:944–954. https://doi.org/10.1016/j.ijbiomac.2019.08.068
Khan SA, Siddiqui MF, Khan TA (2020) Ultrasonic-assisted synthesis of polyacrylamide/bentonite hydrogel nanocomposite for the sequestration of lead and cadmium from aqueous phase: equilibrium, kinetics and thermodynamic studies. Ultrason Sonochem 60:104761. https://doi.org/10.1016/j.ultsonch.2019.104761
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vieira, T., Artifon, S.E.S., Cesco, C.T. et al. Chitosan-based hydrogels for the sorption of metals and dyes in water: isothermal, kinetic, and thermodynamic evaluations. Colloid Polym Sci 299, 649–662 (2021). https://doi.org/10.1007/s00396-020-04786-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-020-04786-2