Abstract
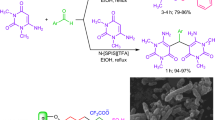
Multi-functional silsesquioxane nanoparticles (SQ-NPs) having two distinct optoelectronic functionalities on a single arm were prepared using a thiol-epoxy click reaction followed by esterification. The epoxy-functionalized SQ-NPs were prepared from commercially available (3-glycidyloxypropyl)triethoxysilane and were employed in the thiol-epoxy click reaction to introduce aromatic and heterocyclic thiol compounds, such as naphthalenethiol, 2-mercapto-1-methylimidazole, and 4,5-diphenyl-2-oxazolethiol. The resulting hydroxyl-functionalized SQ-NPs were further functionalized via esterification to incorporate a second functional group. The X-ray diffraction (XRD), size-exclusion chromatography (SEC), and scanning force microscopy (SFM) results indicated the formation of SQ-NPs (< 5 nm) with relatively narrow size distributions and no aggregation. Multi-functional SQ-NPs containing peripheral electron-accepting benzothiazole moieties were also synthesized using 2-mercaptobenzothiazole. The resulting SQ-NPs showed good solubility, high refractive indices (1.55–1.62), high thermal stability (Td5 > 300 °C), and characteristic optoelectronic properties with a wide range of Stokes shifts (5200–12,000 cm−1). The optoelectronic properties of the multi-functional SQ-NPs could be controlled by modifying the structure of the two distinct functional groups, which could be easily tuned by varying the structure of the thiol compounds and acid chloride derivatives in the feed.







Similar content being viewed by others
References
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081–2173
Gunawidjaja R, Huang F, Gumenna M, Klimenko N, Nunnery GA, Shevchenko V, Tannenbaum R, Tsukruk VV (2009) Bulk and surface assembly of branched amphiphilic polyhedral oligomer silsesquioxane compounds. Langmuir 25:1196–1209
Perez-Ojeda ME, Trastoy B, Rol A, Chiara MD, Garcia-Moreno I, Chiara JL (2013) Controlled click-assembly of well-defined hetero-bifunctional cubic silsesquioxanes and their application in targeted bioimaging. Chem Eur J 19:6630–6640
Olivero F, Reno F, Carniato F, Rizzi M, Cannas M, Marchese L (2012) A novel luminescent bifunctional POSS as a molecular platform for biomedical applications. Dalton Trans 41:7467–7473
Yang X, Froehlich JD, Chae HS, Li S, Mochizuki A, Jabbour GE (2009) Efficient light-emitting devices based on phosphorescent polyhedral oligomeric silsesquioxane materials. Adv Funct Mater 19:2623–2629
Liu D, Yu B, Jiang X, Yin J (2013) Responsive hybrid microcapsules by the one-step interfacial thiol-ene photopolymerization. Langmuir 29:5307–5314
Mori H, Sada C, Konno T, Koizumi R, Yonetake K (2012) Film-forming amphiphilic silsesquioxane hybrids prepared by hydrolytic co-condensation of hydroxyl-functionalized and fluorinated triethoxysilanes. Polymer 53:3849–3860
Li Y, Guo K, Su H, Li X, Feng X, Wang Z, Zhang W, Zhu S, Wesdemiotis C, Cheng SZD, Zhang WB (2014) Tuning “thiol-ene” reactions toward controlled symmetry breaking in polyhedral oligomeric silsesquioxanes. Chem Sci 5:1046–1053
Koizumi R, Kimura T, Nakabayashi K, Mori H (2017) Amphiphilic silsesquioxane nanoparticles by hydrolytic condensation of Y-shaped triethoxysilanes having hydroxyl and fluoroalkyl groups: synthesis, self-assembly, and surface properties. Polymer 110:260–272
Kimura T, Sobu S, Nakabayashi K, Mori H (2017) Dual-functional fluorinated-thiolated silsesquioxane nanoparticles for UV nanoimprinting via thiol-ene chemistry. Polymer 122:60–70
Takeuchi H, Konno T, Mori H (2017) Synthesis of multifunctional silsesquioxane nanoparticles with hydroxyl and polymerizable groups for UV-curable hybrid coating. React Funct Polym 115:43–52
Shibasaki S, Sasaki Y, Nakabayashi K, Mori H (2016) Synthesis and metal complexation of dual-functionalized silsesquioxane nanoparticles by sequential thiol-epoxy click and esterification reactions. React Funct Polym 107:11–19
De S, Khan A (2012) Efficient synthesis of multifunctional polymers via thiol-epoxy "click" chemistry. Chem Commun 48:3130–3132
Gadwal I, Stuparu MC, Khan A (2015) Homopolymer bifunctionalization through sequential thiol–epoxy and esterification reactions: an optimization, quantification, and structural elucidation study. Polym Chem 6:1393–1404
De S, Stelzer C, Khan A (2012) A general synthetic strategy to prepare poly(ethylene glycol)-based multifunctional copolymers. Polym Chem 3:2342–2345
Binder S, Gadwal I, Bielmann A, Khan A (2014) Thiol-epoxy polymerization via an AB monomer: synthetic access to high molecular weight poly(beta-hydroxythio-ether)s. J Polym Sci A Polym Chem 52:2040–2046
Braendle A, Khan A (2012) Thiol-epoxy ‘click’ polymerization: efficient construction of reactive and functional polymers. Polym Chem 3:3224–3227
Gadwal I, Khan A (2013) Protecting-group-free synthesis of chain-end multifunctional polymers by combining ATRP with thiol-epoxy ‘click’ chemistry. Polym Chem 4:2440–2444
Gadwal I, Khan A (2015) Multiply functionalized dendrimers: protective-group-free synthesis through sequential thiolepoxy 'click' chemistry and esterification reaction. RSC Adv 5:43961–43964
Gadwal I, Binder S, Stuparu MC, Khan A (2014) Dual-reactive hyperbranched polymer synthesis through proton transfer polymerization of thiol and epoxide groups. Macromolecules 47:5070–5080
Li S, Han J, Gao C (2013) High-density and hetero-functional group engineering of segmented hyperbranched polymers via click chemistry. Polym Chem 4:1774–1787
Jian Y, He Y, Sun Y, Yang H, Yang W, Nie J (2013) Thiol-epoxy/thiol-acrylate hybrid materials synthesized by photopolymerization. J Mater Chem C 1:4481–4489
Lin H, Ou J, Liu Z, Wang H, Dong J, Zou H (2015) Thiol-epoxy click polymerization for preparation of polymeric monoliths with well-defined 3D framework for capillary liquid chromatography. Anal Chem 87:3476–3483
Lin H, Chen L, Ou J, Liu Z, Wang H, Dong J, Zou H (2015) Preparation of well-controlled three-dimensional skeletal hybrid monoliths via thiol-epoxy click polymerization for highly efficient separation of small molecules in capillary liquid chromatography. J Chromatogr A 1416:74–82
Mazurek P, Daugaard AE, Skolimowski M, Hvilsted S, Skov AL (2015) Preparing mono-dispersed liquid core PDMS microcapsules from thiol-ene-epoxy-tailored flow-focusing microfluidic devices. RSC Adv 5:15379–15386
Ware T, Simon D, Hearon K, Kang TH, Maitland DJ, Voit W (2013) Thiol-click chemistries for responsive neural interfaces. Macromol Biosci 13:1640–1647
Biggs CI, Packer C, Hindmarsh S, Walker M, Wilson NR, Rourke JP, Gibson MI (2017) Impact of sequential surface-modification of graphene oxide on ice nucleation. Phys Chem Chem Phys 19:21929–21932
Acar SB, Ozcelik M, Uyar T, Tasdelen MA (2017) Polyhedral oligomeric silsesquioxane-based hybrid networks obtained via thiol-epoxy click chemistry. Iran Polym J 26:405–411
Toyooka T, Chokshi H, Carlson R, GivensI R, Lunte S (1993) Oxazole-based tagging reagents for analysis of secondary-amines and thiols by liquid-chromatography with fluorescence detection. Analyst 118:257–263
Zhang L, Xu Q, Lu J, Xia X, Wang L (2007) ATRP of MMA initiated by 2-bromomethyl-4,5-diphenyloxazole at room temperature and study of fluorescent property. Eur Polym J 43:2718–2724
Xing Z-H, Zhuang J-Y, Xu X-P, Ji S-J, Su W-M, Cui Z (2017) Novel oxazole-based emitters for high efficiency fluorescent OLEDs: synthesis, characterization, and optoelectronic properties. Tetrahedron 73:2036–2042
Wang Y, Wei Y, Dong C (2006) Study on the interaction of 3,3-bis(4-hydroxy-1-naphthyl)-phthalide with bovine serum albumin by fluorescence spectroscopy. J Photochem Photobiol A Chem 177:6–11
Saltiel J, Sears D, Choi J, Sun Y, Eaker D (1994) Fluorescence, fluorescence excitation, and ultraviolet-absorption spectra of trans-1-(2-naphthyl)-2-phenylethene conformers. J Phys Chem 98:35–46
Singh RB, Mahanta S, Bagchi A, Guchhait N (2009) Interaction of human serum albumin with charge transfer probe ethyl ester of N,N-dimethylamino naphthyl acrylic acid: an extrinsic fluorescence probe for studying protein micro-environment. Photochem Photobiol Sci 8:101–110
Lin Y, Fan H, Li Y, Zhan X (2012) Thiazole-based organic semiconductors for organic electronics. Adv Mater 24:3087–3106
Sheen Y-C, Lu C-H, Huang C-F, Kuo S-W, Chang F-C (2008) Synthesis and characterization of amorphous octakis-functionalized polyhedral oligomeric silsesquioxanes for polymer nanocomposites. Polymer 49:4017–4024
Laine RM, Roll MF (2011) Polyhedral phenylsilsesquioxanes. Macromolecules 44:1073–1109
Liu C, Liu Y, Shen Z, Xie P, Dai D, Zhang R, He C, Chung T (2001) Synthesis and characterization of novel alcohol-soluble ladderlike poly(silsesquioxane)s containing side-chain hydroxy groups. Macromol Chem Phys 202:1576–1580
Liu J-G, Ueda M (2009) High refractive index polymers: fundamental research and practical applications. J Mater Chem 19:8907–8919
Lu C, Yang B (2009) High refractive index organic-inorganic nanocomposites: design, synthesis and application. J Mater Chem 19:2884–2901
Mori H, Takahashi K, Koizumi R, Ohmori K, Hidaka M (2013) Sulfur-containing silsesquioxane hybrids with high refractive index and high abbe number. Colloid Polym Sci 291:1085–1094
Choi J-K, Lee D-H, Rhee S-K, Jeong H-D (2010) Observation of tunable refractive indices and strong intermolecular interactions in newly synthesized methylene-biphenylene-bridged silsesquioxane thin films. J Phys Chem C 144:14233–14239
Tanaka K, Yamane H, Mitamura K, Watase S, Matsukawa K, Chujo Y (2014) Transformation of sulfur to organic-inorganic hybrids employed by POSS networks and their application for the modulation of refractive indices. J Polym Sci A Polym Chem 52:2588–2595
Chen Y-H, Lin L-Y, Lu C-W, Lin F, Huang Z-Y, Lin H-W, Wang PH, Liu YH, Wong KT, Wen J, Miller DJ, Darling SB (2012) Vacuum-deposited small-molecule organic solar cells with high power conversion efficiencies by judicious molecular design and device optimization. J Am Chem Soc 134:13616–13623
Kato S-I, Matsumoto T, Shigeiwa M, Gorohmaru H, Maeda S, Ishi-i T et al (2006) Novel 2,1,3-Benzothiadiazole-based red-fluorescent dyes with enhanced two-photon absorption cross-sections. Chem A Eur J 3:2303–2317
Alfonso M, Espinosa A, Tárraga A, Molina P (2014) Multifunctional benzothiadiazole-based small molecules displaying solvatochromism and sensing properties toward nitroarenes, anions, and cations. Chem Open 3:242–249
Neto BAD, Lapis AAM, da Silva Júnior EN, Dupont J (2013) 2,1,3-Benzothiadiazole and derivatives: synthesis, properties, reactions, and applications in light technology of small molecules. Eur J Org Chem 2013:228–255
Sonar P, Singh SP, Leclere P, Surin M, Lazzaroni R, Lin TT et al (2009) Synthesis, characterization and comparative study of thiophene-benzothiadiazole based donor-acceptor-donor (D-A-D) materials. J Mater Chem 19:3228–3237
Zhang M, Tsao HN, Pisula W, Yang C, Mishra AK, Müllen K (2007) Field-effect transistors based on a benzothiadiazole-cyclopentadithiophene copolymer. J Am Chem Soc 129:3472–3473
Liu X, Hsu BBY, Sun Y, Mai C-K, Heeger AJ, Bazan GC (2014) High thermal stability solution-processable narrow-band gap molecular semiconductors. J Am Chem Soc 136:16144–16147
Pina J, JSd M, Breusov D, Scherf U (2013) Donor–acceptor–donor thienyl/bithienyl-benzothiadiazole/quinoxaline model oligomers: experimental and theoretical studies. Phys Chem Chem Phys 15:15204–15213
Fu B, Baltazar J, Hu Z, Chien A-T, Kumar S, Henderson CL, Collard DM, Reichmanis E (2012) High charge carrier mobility, low band gap donor−acceptor benzothiadiazole-oligothiophene based polymeric semiconductors. Chem Mater 24:4123–4133
Funding
There was no financial support obtained for the reported work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1117 kb)
Rights and permissions
About this article
Cite this article
Sasaki, Y., Shibasaki, S., Lo, CT. et al. Design and synthesis of multi-functional silsesquioxane nanoparticles having two distinct optoelectronic functionalities. Colloid Polym Sci 296, 1017–1028 (2018). https://doi.org/10.1007/s00396-018-4320-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4320-0