Abstract
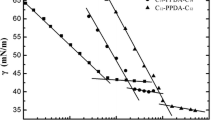
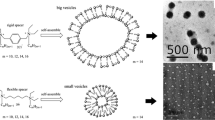
New series of ester functionalized quaternary ammonium gemini surfactants having different ethylene oxide units as spacer have been synthesized and investigated for their aggregation behavior and thermodynamic properties of micellization by surface tension, conductivity, and fluorescence methods. The critical micelle concentration (cmc) of these gemini surfactants increases with the increase in the length of polar hydrophilic ethylene oxide spacer. The micellization process has been found to be entropy-driven and dependent on both the tendency of the hydrophobic group of the surfactants to transfer from aqueous environment to interior of micelle as well as the rearrangement of flexible ester-linked ethylene oxide units (hydrophilic spacer) into aqueous phase. The polar ester functional groups and pairs of nonbonding electrons on oxygen atom of ethylene oxide spacer form hydrogen bonding with water molecules enhancing their solubility in aqueous system.





Similar content being viewed by others
References
Zana R, Xia J (2004) Gemini surfactants: synthesis, interfacial and solution phase behavior, and application. Marcel Dekker, Inc
Menger FM, Littau CA (1991) J Am Chem Soc 113:1451–1452
Tehrani-Bagha AR, Holmberg K (2010) Langmuir 26:9276–9282
Huang X, Han Y, Wang Y, Cao M, Wang Y (2008) Colloids Surf A Physicochem Eng Asp 325:26–32
Chlebicki J, Wegrzynska J, Wilk KA (2008) J Colloid Interface Sci 323:372–378
Banno T, Toshima K, Kawada K, Matsumura S (2009) J Surfactant Deterg 12:249–259
Rist Ø, Rike A, Ljone L, Carlsen PHJ (2001) Molecules 6:979–987
Sharma VD, Ilies MA (2014) Med Res Rev 34:1–44
Wettig SD, Li X, Verrall RE (2003) Langmuir 19:3666–3670
Klimavicius V, Gdaniec Z, Kausteklis J, Aleksa V, Aidas K, Balevicius V (2013) J Phys Chem B 117:10211–10220
Katsyuba SA, Vener MV, Zvereva EE, Fei Z, Scopelliti R, Laurenczy G, Yan N, Paunescu E, Dyson PJ (2013) J Phys Chem B 117:9094–9105
Obrzud M, Rospenk M, Koll A (2014) Phys Chem Chem Phys 16:3209
Narayanan C, Dias CL (2013) J Chem Phys 139:115103
Bhadani A, Singh S, Kamboj R, Chauhan V (2013) Colloid Polym Sci 291:2289–2297
Gaikar VG (2004) Chem Ind Dig 17:54–55
Matsumura S (2002) Eco Ind 8:12–23
Tehrani-Bagha AR, Oskarsson H, van Ginkel CG, Holmberg K (2007) J Colloid Interface Sci 312:444–452
Brown P, Bushmelev A, Butts CP, Cheng J, Eastoe J, Grillo I, Heenan RK, Schmidt AM (2012) Angew Chem Int Ed 51:2414–2416
Sakai K, Umezawa S, Tamura M, Takamatsu Y, Tsuchiya K, Torigoe K, Ohkubo T, Yoshimura T, Esumi K, Sakai H, Abe M (2008) J Colloid Interface Sci 318:440–448
Fisicaro E, Compari C, Biemmi M, Duce E, Peroni M, Barbero N, Viscardi G, Quagliotto P (2008) J Phys Chem B 112:12312–12317
Pinazo A, Wen X, Perez L, Infante MR, Franses EI (1999) Langmuir 15:3134
Tsubone K, Arakawa Y, Rosen MJ (2003) J Colloid Interface Sci 26:2516
Alami E, Beinert G, Marie P, Zana R (1993) Langmuir 9:1465–1467
Venkatesan P, Cheng Y, Kahne D (1994) J Am Chem Soc 116:6955–6956
Quagliotto P, Viscardi G, Barolo C, Barni E, Bellinvia S, Fisicaro E, Compari C (2003) J Org Chem 68:7651–7660
Zhuang L-H, Yu K-H, Wang G-W, Yao C (2013) J Colloid Interface Sci 408:94–100
Ao M, Huang P, Xu G, Yang X, Wang Y (2009) Colloid Polym Sci 287:395–402
Ao M, Xu G, Zhu Y, Bai Y (2008) J Colloid Interface Sci 326:490–495
Cai B, Li X, Yang Y, Dong J (2012) J Colloid Interface Sci 370:111–116
Zana R (1996) Langmuir 12:1210
Li H, Yu C, Chen R, Li J, Li J (2012) Colloids Surf A: Physicochem Eng Asp 395:118
Zhang Q, Gao Z, Xu F, Tai S, Liu X, Mo S, Ni F (2012) Langmuir 28:11979–11987
Acknowledgments
A. B is thankful to Tokyo University of Science, Tokyo, Japan, for the Postdoctoral Research Fellowship and research support.
Author information
Authors and Affiliations
Corresponding authors
Supplementary material
Below is the link to the electronic supplementary material.
396_2014_3233_MOESM1_ESM.docx
Spectral data of initial bromoesters. 1H and 13C NMR spectra of the all the molecules synthesized in the present work. (DOCX 422 kb)
Rights and permissions
About this article
Cite this article
Bhadani, A., Endo, T., Sakai, K. et al. Synthesis and dilute aqueous solution properties of ester functionalized cationic gemini surfactants having different ethylene oxide units as spacer. Colloid Polym Sci 292, 1685–1692 (2014). https://doi.org/10.1007/s00396-014-3233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3233-9