Abstract
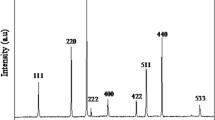
Cobalt–iron cyanide (Cox[Fe(CN)6]) nanoparticles have been synthesized by a novel solid–solid reaction in the confined space of dry sodium bis(2-ethylhexyl)sulfosuccinate (AOT) reversed micelles dispersed in n-heptane. The reaction has been carried out by mixing two dry AOT/n-heptane solutions containing CoCl2 and K4Fe(CN)6 or K3Fe(CN)6 nanoparticles in the micellar core, respectively. By UV-Vis spectroscopy it was ascertained that, after the mixing process, the formation of stable nanoparticles is fast and complete. Microcalorimetric measurements of the thermal effect due to the Cox[Fe(CN)6] nanoparticle formation allowed the determination of the stoichiometric ratio (x) and of the molar enthalpy of reaction in the core of AOT reversed micelles. The observed behavior suggests the occurrence of confinement effects and surfactant adsorption on the nanoparticle surface. Further structural information was achieved by small-angle X-ray scattering (SAXS) measurements. From all liquid samples, interesting salt/AOT composites were prepared by simple evaporation of the apolar solvent. Size, crystal structure, and electronic properties of Cox[Fe(CN)6] nanoparticles containing composites were obtained by wide-angle X-ray scattering (WAXS) and X-ray photoelectron spectroscopy (XPS).















Similar content being viewed by others
References
Calandra P, Longo A, Turco Liveri V (2003) J Phys Chem B 107:25–30
Calandra P, Longo A, Marcianò V, Turco Liveri V (2003) J Phys Chem B 107:6724–6729
Turco Liveri V (1999) Curr Top Colloids Interface Sci 3:65–74
Calandra P, Longo A, Turco Liveri V (2001) Colloid Polym Sci 279:1112–1117
Chow PY, Ding J, Wang XZ, Chew CH, Gan LM (2000) Phys Stat Sol A 180:547–553
Vesta CR, Zhang ZH (2002) Chem Mater 14:3817–3822
Ruland W (1974) J Appl Cryst 7:383–386
Feign LA, Svergun DI (1987) Structure analysis by small angle X-ray and neutron scattering. Plenum Press, New York
Giordano C, Longo A, Turco Liveri V, Venezia AM (2003) Colloid Polym Sci 281:229–238
Kitahara A, Kon-no K (1966) J Phys Chem 70:3394–3398
Nitsch W, Plucinski P (1990) J Coll Int Sci 136:338–351
Linke WF, Seidell A (1958) Solubilities of inorganic and metal organic coumpounds, vol 1. Van Nostrand, New York
Handbook of chemistry and physics, 67th edn (1986–1987) CRC Press, Boca Raton, FL
Eastoe J, Stebbing S, Dalton J, Heenan R (1996) Colloid Surf A 119:123–131
Sunamoto J, Hamada T (1978) Bull Chem Soc Jpn 51:3130–3135
Nicholls D (1973) Compr Inorg Chem 3:1025–1051
Naiman CS (1961) J Chem Phys 35:323–328
Bonner OD, Choi YS (1974) J Phys Chem 78:1723–1727
Onori G, Santucci A (1993) J Phys Chem 97:5430–5434
North AN, Dore JC, McDonald JA, Robinson BH, Heenan RK, Howe AM (1986) Colloids Surf 19:21–29
Mackeben S, Müller-Goymann CC (2000) Int J Pharm 196:207–210
Kotlarchyk M, Huang JS, Chen SH (1985) J Phys Chem 89:4382–4386
Hirai M, Kawai-Hirai R, Yabuki S, Takizawa T, Hirai T, Kobayashi K, Amemiya Y, Oya M (1995) J Phys Chem 99:6652–6660
Ekwall P, Mandell L, Fontell K (1970) J Coll Int Sci 33:215–235
Chastain (ed) (1992) Handbook of X-ray photoelectron spectroscopy. Perkin–Elmer, Eden Prairie, MN
Acknowledgements
Financial support from Università di Palermo is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giordano, C., Longo, A., Ruggirello, A. et al. Physicochemical investigation of cobalt–iron cyanide nanoparticles synthesized by a novel solid–solid reaction in confined space. Colloid Polym Sci 283, 265–276 (2004). https://doi.org/10.1007/s00396-004-1124-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1124-1