Abstract
Short repeated cycles of peripheral ischemia/reperfusion (I/R) can protect distant organs from subsequent prolonged I/R injury; a phenomenon known as remote ischemic preconditioning (RIPC). A RIPC-mediated release of humoral factors might play a key role in this protection and vascular endothelial cells are potential targets for these secreted factors. In the present study, RIPC-plasma obtained from healthy male volunteers was tested for its ability to protect human umbilical endothelial cells (HUVEC) from hypoxia–induced cell damage. 10 healthy male volunteers were subjected to a RIPC-protocol consisting of 4 × 5 min inflation/deflation of a blood pressure cuff located at the upper arm. Plasma was collected before (T0; control), directly after (T1) and 1 h after (T2) the RIPC procedure. HUVEC were subjected to 24 h hypoxia damage and simultaneously incubated with 5 % of the respective RIPC-plasma. Cell damage was evaluated by lactate dehydrogenase (LDH)-measurements. Western blot experiments of hypoxia inducible factor 1 alpha (HIF1alpha), phosphorylated signal transducer and activator of transcription 5 (STAT5), protein kinase B (AKT) and extracellular signal-related kinase 1/2 (ERK-1/2) were performed. Furthermore, the concentrations of hVEGF were evaluated in the RIPC-plasma by sandwich ELISA. Hypoxia–induced cell damage was significantly reduced by plasma T1 (p = 0.02 vs T0). The protective effect of plasma T1 was accompanied by an augmentation of the intracellular HIF1alpha (p = 0.01 vs T0) and increased phosphorylation of ERK-1/2 (p = 0.03 vs T0). Phosphorylation of AKT and STAT5 remained unchanged. Analysis of the protective RIPC-plasma T1 showed significantly reduced levels of hVEGF (p = 0.01 vs T0). RIPC plasma protects endothelial cells from hypoxia–induced cell damage and humoral mediators as well as intracellular HIF1alpha may be involved.
Similar content being viewed by others
Introduction
Transient episodes of ischemia (ischemic preconditioning), if applied before prolonged ischemia/reperfusion injury, are organ protective [12, 26]. Ischemic preconditioning does not only act locally, but is also able to protect remote tissues from ischemia/reperfusion injury, a phenomenon described as remote ischemic preconditioning (RIPC). RIPC can be induced by inflation and deflation of a blood pressure cuff located at the upper or lower limb. This procedure has been shown to attenuate organ injury in a number of experimental and clinical situations [26, 27, 58].
Despite the encouraging results of RIPC in preclinical and animal studies, a translation of RIPC into the clinic is still not fully achieved [2, 16, 49, 57]. However, there are several clinical trials in progress elucidating the potential clinical benefit of RIPC. In patients undergoing coronary artery bypass graft (CABG) surgery an acute and probably favourable attenuation of cardiac enzyme release has been demonstrated, and thus there is evidence that RIPC provides perioperative myocardial protection and improves the prognosis of these patients [9, 68, 69]. Similar protective effects could also be found in patients undergoing elective percutaneous coronary intervention (PCI) [14, 34] and ST-elevation myocardial infarction (STEMI) patients undergoing primary PCI [5, 65]. On the other hand, deleterious effects with a significant increase in cardiac injury have been described [10, 37, 59]. Therefore, the results of the large clinical trials ongoing in CABG patients [22, 50] will substantially increase our knowledge about the translatability of RIPC in the clinical setting.
The underlying mechanisms of RIPC have been attributed to humoral, neuronal and anti-inflammatory pathways [27]. However, the exact mechanisms are complex and yet not completely understood [24, 62]. It is suggested that the humoral mediators might be released from the remote tissue into the blood stream from where they are transported to the target organ, and/or that they are produced after stimulation via neuronal pathways in the target organ itself [24, 47]. In the last years, several so far unidentified mediators of RIPC have been described by different groups: e.g. stomal derived factor (SDF) 1alpha [6], exosomes [19], Apolipoprotein A1 [32], miR144 [45], IL-10 [8], matrix metalloproteinases (MMPs) [74] and nitrite [60]. For review, also see [27].
For several reasons the vascular endothelium, especially in the target organ, could play a central role in the RIPC-mediated mechanisms of organ protection from I/R injury: (1) humoral factors that are released into the blood stream upon RIPC stimulus may directly interact with endothelial cells which in turn may directly or indirectly transfer the RIPC stimulus to the underlying tissue [51]. (2) Endothelial cells are among the first cell types that will encounter hypoxia in the target organ and respond to it [63]. (3) Endothelial dysfunction is a major reason for severe local and systemic consequences of I/R injury [63]. Moreover, it has been demonstrated that RIPC before primary percutaneous coronary intervention significantly improves endothelial function in patients with acute myocardial infarction, and this effect remains constant for at least a week [48]. These data suggest that the improvement of endothelial function may be one possible explanation for the protective effects of RIPC. In this context it is worth mentioning that the coronary circulation and cardiac remodelling that are directly related to the endothelial function just recently have been recognized to be critical determinants of cardioprotective interventions [28, 29].
In the present study, RIPC-plasma was obtained from healthy male volunteers and tested for its ability to protect human vascular endothelial cells (HUVEC) from hypoxia–induced cell damage. We furthermore investigated the cellular target mechanisms that were affected by RIPC-plasma in HUVEC cells subjected to a hypoxic insult.
Materials and methods
Chemicals, solutions and culture media
If not otherwise stated all chemicals and solutions were purchased either from Roche (Almere, the Netherlands), Sigma-Aldrich (Zwijndrecht, Netherlands), Merck (Millipore, Amsterdam, Netherlands) or Carl Roth (Karlsruhe, Germany).
Isolation of human umbilical vein endothelial cells (HUVEC)
HUVEC were freshly isolated from umbilical cords as described previously [71] (Waiver: W12-167#12.17.096, Ethical committee Amsterdam) and maintained in a humidified atmosphere of 5 % carbon dioxide/95 % air at 37 °C in ECGM medium supplemented with endothelial cell growth supplement (Promocell Bio-Connect, Huizen, Netherlands), 1 % penicillin/streptomycin, 1 % amphotericin B and 10 % heat-inactivated foetal bovine serum (PAA, Germany, Freiburg). All culture surfaces were coated with 0.75 % gelatine (BD Diagnostic Systems, Netherlands, Breda) prior to cell seeding. Only cells from passage 3 were used in the experiments. Characterization of HUVEC cells was performed by detection of von Willebrand-factor by fluorescence staining and fluorescence activated cell sorting FACS (data not shown).
Collection of human plasma
The study was approved by the local ethics committee of the Academic Medical Centre (AMC), University of Amsterdam, The Netherlands, (ISRCTN59201440) and was performed in accordance with the Declaration of Helsinki and the Medical Research Involving Human Subjects Act. Ten healthy male volunteers (25.20 ± 3.39 years) were subjected to a RIPC-protocol consisting of 4 × 5 min inflation/deflation of a blood pressure cuff located at the upper right arm. Prior to participation, all subjects gave their written informed consent. The following inclusion criteria were chosen: (1) healthy, (2) male, (3) age between 18 AND 45 years. Exclusion criteria were as follows: (1) cardiovascular, kidney, pulmonary or endocrine diseases, (2) alcohol or drug abuse, and (3) no informed consent. We decided to only include male volunteers into the study to avoid potential influences of oestrogens [56]. Subject characteristics are given in Table 1.
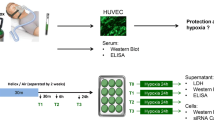
Blood was collected before (T0; baseline time point prior to inflation of blood pressure cuff = controls), directly after (T1) and 60 min after (T2) the RIPC-protocol (Fig. 1). The blood samples were collected in citrate vials (BD bioscience, Breda, The Netherlands) and centrifuged at 4 °C, 290 g for 10 min. Plasma was aliquoted, and stored at −80 °C.
Experimental setting. The RIPC-protocol consisted of 4 × 5 min inflation/deflation of a blood pressure cuff. Plasma was obtained before (T0), directly after (T1) and 60 min after (T2) the RIPC stimulus. HUVEC cells were incubated with the respective plasma and subjected to 24 h of hypoxia. Employing cell culture media and cell lysates, cell damage as well as cellular signalling events were investigated. H hypoxia; green color, RIPC-plasma
To evaluate the optimal concentration of human plasma to be added to the culture medium, HUVEC cells were incubated with different concentrations (0, 1, 5, 10 %) of fetal bovine serum and growth characteristics as well as the degree of hypoxia–induced cell damage were evaluated. On the basis of these results, the optimal concentration of plasma was selected. A similar approach has also been chosen in our recently published study in which RIPC serum was used [74]. These evaluations suggested that the basic culture medium should be supplemented with 5 % plasma.
Induction of in vitro hypoxia in HUVEC by enzymatic oxygen depletion
Hypoxic conditions in HUVEC were induced using a modified version of our recently described enzymatic model [36, 75]. In the present study concentrations of the hypoxia inducing enzymes were adapted to 4 U/ml glucose oxidase (GO), and 120 U/ml catalase (CAT) and cell culture plates were transferred into an airtight chamber, which was flooded with 1 bar, 10 l/min nitrogen gas until oxygen was reduced to 1 %. The box was then kept inside an incubator at 37 °C. Oxygen levels were monitored directly in the culture medium using an OxyMini fibre optic oxygen meter (World Precision Instruments, USA, Sarasota) and OxyMicro Software v.00 04/2003.
Experimental protocol
HUVEC cells were seeded into gelatine-coated 12 well plates 2 days before the experiment. At the beginning of the experiment ECGM growth medium was exchanged and cells were pre-incubated for 1 h with M199 medium (PAN Biotech, Aidenbach, Germany) containing 5 % of the respective RIPC-plasma (T0, T1, T2). Subsequently, the normoxic medium was replaced by hypoxic M199 containing 5 % of the respective RIPC-plasma and the culture plates were placed in an airtight chamber and kept inside an incubator for 24 h. For colorimetric lactate dehydrogenase (LDH)-measurements the cell culture medium of each well was collected after 24 h of hypoxia and frozen at −20 °C. For Western blotting experiments the cells were harvested and frozen at −80 °C (Fig. 1).
Determination of cell death
LDH activity was colorimetrically evaluated using a Lactate Dehydrogenase Activity Assay Kit (Biovision, Uithoorn, Netherlands,) following the manufacturer’s protocol. Absorbance was measured before and after 30 min of incubation (37 °C) at 450 nm using an ELISA reader (Tecan, Crailsheim, Germany).
Western blotting
Western blotting was performed as described previously [74]. Overnight incubation of the membranes was conducted at 4 °C with appropriate dilutions of primary antibodies directed against HIF1alpha (Acris, Novus Biological, Herford, Germany, 1:1000), actin (Santa Cruz, Heidelberg, Germany, 1:1000), pAKT (Cell Signalling, Danvers, USA, 1:1000), AKT (Cell Signalling, Danvers, USA, 1:2000), pERK-1/2 (Cell Signalling, Danvers, USA, 1:8000), ERK-1/2 (Cell Signalling, Danvers, USA, 1:8000), pSTAT5 (R&D Systems, Wiesbaden-Nordenstadt, Germany, 1:1000), or against STAT5 (R&D Systems, Wiesbaden-Nordenstadt, Germany, 1:1000). Membranes were rinsed three times for 10 min with tris-buffered saline plus tween buffer (TBST) at room temperature, and were—depending on the primary antibody used—incubated for 1 h with the secondary horseradish peroxidase coupled-antibody (anti-rabbit, DAKO, Eching, Germany, 1:10,000 or anti-goat, Santa Cruz, Heidelberg, Germany, 1:10,000), with a biotin coupled-antibody (anti-rabbit, Abcam, Cambridge, UK, 1:10,000), or with horseradish peroxidase-coupled streptavidin for the biotinylated secondary antibody (AbD Serotec, Puchheim, Germany, 1:5000). After washing three times with TBST buffer for 10 min, the membranes were incubated with ECL detection reagent (GE Healthcare Life Sciences, Freiburg, Germany) for 5 min. For signal detection, autoradiography films (GE Healthcare Life Sciences, Freiburg, Germany) were exposed to the membranes for various time periods in the dark. Relative intensities of protein bands were analysed by ImageJ v1.48 and GraphPad Prism 5.0 for Mac. In some experiments, membranes were stripped and re-probed with different antibodies. Therefore, the membranes were incubated for 15 min at 56 °C with stripping buffer (4 ml SDS 10 %, 2.5 ml Tris 0.5 M pH 6.8, 13.5 ml ultrapure water, 160 µl 2-mercaptoethanol). After washing with TBST buffer, the membranes were re-blocked with 3 % BSA/TBST buffer for 1 h and were washed three times with TBST buffer.
Quantification of human vascular endothelial growth factor
The concentrations of the human Vascular Endothelial Growth Factor (hVEGF) were determined in RIPC-plasma by specific ELISA systems, (ScienceCell Research Laboratories, USA, Carlsbad) according to the manufacturer’s protocol. Absorbance was measured at 450 nm using an ELISA reader (Tecan, Germany, Crailsheim) and hVEGF concentrations were calculated from the standard curve provided.
Statistical analysis
Statistics were performed using the software GraphPad Prism 5.0 for Mac. D’Agostino normality testing was used to check data for normal distribution. Parametric data were analysed using One-Sample t-Tests (LDH-activity, hVEGF) or Paired t-Tests (HIF1alpha, pSTAT5). Non-parametric data were analysed using the Wilcoxon signed rank test (pAKT, pERK-1/2). Variables are expressed as mean ± SEM.
Results
Plasma obtained directly after RIPC reduces the hypoxia–induced cell damage in HUVEC cells
LDH-assays were used to evaluate the influence of RIPC-plasma (T1, T2) on hypoxia–induced cell damage of HUVEC cells. T0-plasma, obtained prior to RIPC, was used as baseline control. Mean LDH-activity in the culture media at baseline was 7.58 ± 0.9 mU/ml, while the range of LDH activity varied between different samples (minimum LDH: 1.50 mU/ml, maximum LDH: 15.24 mU/ml; Fig. 2a) Compared to plasma T0, plasma T1 significantly reduced the hypoxia–induced cell damage (T1: 0.89 ± 0.04; T0 = 1; p = 0.02; Fig. 2b). Plasma T2 did not significantly change the hypoxia induced damage in HUVEC cells (T2: 1.04 ± 0.03; T0 = 1; p = 0.15; Fig. 2b).
Effects of RIPC-plasma on hypoxia–induced damage of HUVEC cells. a Quantification of LDH-activities as a marker for cell damage in HUVEC cell culture media. Culture media were supplemented with 5 % plasma (T0, T1 and T2) from the various volunteers (#1–#10) and LDH-activities were measured after 24 h of hypoxia. b Relative LDH-activities in culture media of HUVEC cells after 24 h of hypoxia. Cell culture media were supplemented with plasma T0, T1 or T2. Hypoxia–induced cell damage is significantly reduced by the addition of plasma T1. Numbers in the columns show the numbers of different plasma samples used. Columns display the mean ± SEM
The reduction of hypoxia–induced cell damage by RIPC-plasma is associated with an increased protein expression of HIF1alpha and enhanced phosphorylation of ERK-1/2
Using Western blotting, the expression of several proteins potentially involved in RIPC-mediated organ protection was investigated [21, 24, 39]. As only plasma T1 but not T2 was able to significantly reduce hypoxia–induced cell damage in HUVEC cells (Fig. 2), all Western blotting experiments were performed with HUVEC cells incubated with T1 and the respective control plasma (T0). Densitometric analyses revealed no statistically significant differences in the phosphorylation of AKT in HUVEC cells that were incubated with plasma T1 (T1: 0.12 ± 0.04 arbitrary units (a.u.) versus T0: 0.16 ± 0.1 a.u.; p = 0.85; Fig. 3a) or STAT5 (T1: 0.53 ± 0.1 a.u. versus T0: 0.65 ± 0.1 a.u.; p = 0.10; Fig. 3b). However, plasma T1 significantly augmented the amount of HIF1alpha (T1: 0.79 ± 0.2 a.u. versus T0: 0.43 ± 0.1 a.u.; p = 0.01; Fig. 3c) and increased the phosphorylation of ERK-1/2 (T1: 0.66 ± 0.3 a.u. versus T0: 0.29 ± 0.1 a.u.; p = 0.03; Fig. 3d) in HUVEC cells that were exposed to 24 h of hypoxia. Control studies revealed that the significant increase in HIF1alpha expression and the significantly increased phosphorylation of ERK-1/2 that were detected after the addition of protective plasma T1 was not evident when employing the non-protective plasma T2 (data not shown).
Effects of RIPC-plasma on protein expression and phosphorylation in HUVEC cells exposed to hypoxia. a Phosphorylation of AKT; b phosphorylation of STAT5; c expression of HIF1alpha; d phosphorylation of ERK-1/2. e Western blotting experiments performed with lysates of HUVEC cells that were treated with RIPC-plasma samples (#1–#10). Numbers in the columns show the numbers of different plasma samples used. Columns display the mean ± SEM
Concentrations of VEGF are reduced in protective RIPC-plasma T1
As VEGF is discussed to be a humoral factor involved in RIPC-mediated organ protection [11, 13, 55], we evaluated the concentrations of human VEGF (hVEGF) in RIPC plasma using a sandwich ELISA system. Mean hVEGF concentrations in plasma T0 were 67.91 ± 32.9 pg/ml, while the range of hVEGF varied between plasma samples of different donors (minimum hVEGF: 8.26 pg/ml, maximum hVEGF: 352.80 pg/ml; Fig. 4a) Concentrations of hVEGF were significantly reduced in the protective plasma (T1) which was derived directly after RIPC (T1: 0.87 ± 0.04; T0 = 1; p = 0.01; Fig. 4b).
Discussion
The major findings of the present study are the following: (1) human plasma retrieved directly after remote ischemic preconditioning (RIPC) is able to reduce hypoxia–induced damage of human endothelial cells cultured in vitro. (2) Expression of HIF1alpha but not phosphorylation of ERK-1/2, AKT or STAT5 seems to be involved in the protective effects of RIPC-plasma. (3) The protective RIPC-plasma contains decreased amounts of VEGF.
To date, the exact mechanisms of RIPC are not fully understood. However, three hypotheses to explain the phenomenon of remote ischemic organ protection have been established: (1) RIPC triggers the release of humoral factors into the bloodstream from where they reach the remote target organ; (2) neuronal pathways confer the RIPC-protection; and (3) a systemic anti-inflammatory and anti-apoptotic response is induced by the RIPC stimulus [24, 62]. Recently, several circulating mediators have been identified, e.g. stromal derived factor (SDF) 1alpha [6], exosomes [19], Apolipoprotein A1 [32], miR144 [45], IL-10 [8], or nitrite [60] that may be involved in RIPC-mediated cell and organ protection. Using an in vitro approach, we showed that serum from cardiac surgical RIPC patients as well as culture media from hypoxia–conditioned HUVEC cells are both able to reduce hypoxia–induced cell damage in intestinal cell cultures [36, 74]. These results underline the potential role of secreted factors for RIPC-mediated organ protection. Here we extended our recent studies and applied RIPC-plasma, which was retrieved from healthy male volunteers, to cultured endothelial cells.
In our study, plasma from RIPC volunteers (obtained before, directly after and 60 min after RIPC) was added to the HUVEC cell cultures 1 h before the hypoxic insult and cells were incubated with plasma-substituted medium for 24 h. It is known that ischemic preconditioning [42] represents a biphasic phenomenon with a first and a second window of protection [35] and similar mechanisms may also be effective in RIPC. The early phase of protection develops quickly within minutes from the initial ischemic conditioning event and lasts for 2–3 h. This is followed by a delayed phase that begins after 12–24 h and lasts up to 4 days. The mechanisms of the two phases of preconditioning are rather different. While the early phase is caused by rapid release or modification of pre-existing proteins, the delayed phase requires synthesis of new proteins [43, 44].
Our present findings showing cytoprotective effects of RIPC-plasma that was obtained directly after RIPC, but not of plasma derived 60 min after RIPC is somewhat in contrast to the above mentioned studies, clinical observations and also to our previous publication in intestinal cells (subjected to a hypoxic insult) [74]. However, in the frame of our previous study, RIPC sera were collected from mostly older cardiac surgical patients, while in the study presented here, 10 young and healthy donors were investigated. Several authors have shown that age, diet, hormonal status, comorbidities and other factors may influence and modify the protective potential of ischemic conditioning [1, 15, 17, 53]. Furthermore, the observation that only plasma that was derived directly after RIPC protected HUVEC cells from hypoxia–induced cell damage could be related to the half-life of the responsible factor(s). Potential mediators that might transfer the RIPC protection are adenosine [52, 61, 66], bradykinin [38, 61], opioids [67] as well as matrix MMPs [46, 73, 74] for review see [41], all of which have a limited half-life in circulation [54] and cell culture [18] and—especially in the case of MMPs—can be modified and/or degraded by other proteases [6, 73].
It should also be mentioned that while other authors employed serum [74], in the study presented we used plasma from RIPC treated volunteers. Compared to serum, plasma contains clotting factors such as fibrinogen but is deficient of mediators that are released from blood cells (mainly thrombocytes) upon coagulation. There is no evidence that these molecules interfere with RIPC-mediated processes and we therefore do not expect differences in the protective potential of serum in comparison to plasma.
Taken together, although we do not have a clear explanation why plasma obtained 60 min after RIPC was not effective in protecting HUVEC cells from hypoxia–induced cell damage in the study presented, individual characteristics (age, gender, hormonal status, diet, etc.) of the plasma donors and the use of an in vitro culture system (devoid of e.g. immune cells, humoral factors, blood circulation, etc.) may at least partially be responsible for this observation. Moreover, not all aspects of ischemia/reperfusion injury as they appear in vivo can be reflected using this in vitro system. However, the cell culture model enables us to reproducibly investigate isolated events of ischemia/hypoxia and the associated cellular as well as molecular mechanisms, which is probably the biggest advantage over animal and clinical studies.
Regarding the mechanisms that are induced by RIPC in the target cells, we found an increased amount of HIF1alpha protein in hypoxia stressed HUVEC cells that were treated with plasma derived directly after RIPC. HIF1alpha acts as an oxygen-regulated transcription factor controlling oxygen homeostasis [64] and activation of HIF1alpha leads to induction of target gene activation of e.g. erythropoetin, hexokinase 1 and 2, iNOS and VEGF [33]. Although little is known about the precise role of HIF1alpha in RIPC, several studies proposed an involvement of the protein in ischemic preconditioning [7, 25, 64]. However, there is also a study suggesting that upregulation of HIF1alpha in limb is not associated with myocardial protection of early RIPC and might only act locally [40]. Employing cardiac tissue of cardiosurgical patients that received RIPC or sham intervention, we recently showed that HIF1alpha expression was significantly increased in cardiac tissue of RIPC patients [3], pointing towards possible organ protective effects of enhanced HIF1alpha expression. The protective role of HIF1alpha is also supported by our preliminary experiments employing the HIF1alpha inhibitor LY294002 (10 µM) [72] which showed that the cytoprotective effects of plasma T1 are attenuated by inhibiting the translation of HIF1alpha.
Besides an enhanced expression of HIF1alpha, phosphorylation of ERK-1/2 was increased in hypoxia stressed HUVEC cells that were treated with plasma derived directly after RIPC. It is known that phosphorylation of ERK, being part of the pro-survival MAPK/ERK-pathway, results in inhibition of the pro-apoptotic Bad and is thus impeding the process of apoptotic cell death [20, 62]. In HUVEC cells, transient hypoxia can induce anti-apoptotic events and increase cell survival via ERK-dependent pathways [20] and in the porcine heart RIPC effects are also associated with augmented levels of phosphorylated ERK-1/2 [23]. Interestingly, our preliminary data employing the ERK kinase inhibitor PD98059 (10 µM) in combination with the protective plasma T1 did not confirm an involvement of ERK-1/2 in our in vitro setting, as the inhibition of ERK-1/2 phosphorylation did not result in increased cell damage measured by LDH activity. We propose that further work is necessary to elucidate the precise role of ERK-1/2 activation in RIPC-mediated cytoprotection, especially in endothelial cells.
Hausenloy et al. [23] not only reported pERK-1/2 to be involved in RIPC-mediated organ protection, but also pAKT. Similar to pERK-1/2, pAKT is involved in cellular survival pathways: the PI3 K/AKT-pathway, also known as “reperfusion injury salvage kinase (RISK) pathway”, phosphorylates and thereby inactivates the pro-apoptotic Bad leading to an inhibition of apoptosis [39, 62]. Interestingly, in the present study we did not observe an increased phosphorylation of AKT, which might indicate that different cell types (e.g. myocardial cells versus endothelial cells) respond differently to the RIPC stimulus.
In the literature, the involvement of STAT5 in RIPC is discussed controversially. While an increased phosphorylation of STAT5 was described in ventricular cells after RIPC in humans [31], other authors did not find alterations in STAT5 phosphorylation using cultured intestinal cells [36]. From recent studies it appears that STAT3 and STAT5 might have reverse functions in animals and humans: STAT5 but not STAT3 activation is associated with protection in humans [31], whereas STAT3 activation and possibly STAT5 inhibition are associated with protection in animals [30]. In a clinical trial with RIPC patients undergoing coronary artery bypass surgery, Heusch et al. [31] have shown that the phosphorylation of STAT5 increased from baseline before ischemic cardioplegic arrest to 10 min of reperfusion with RIPC, and that STAT5 phosphorylation during reperfusion was greater in patients with RIPC than in control patients. Once more, the target organ and/or target cell type might determine which signalling pathways are induced via RIPC and this might explain the lack of STAT5 phosphorylation in the context of our study.
Concerning possible factors transferring the RIPC signal to the target cells, VEGF could be a potential candidate [11, 13, 55]. Several studies suggested that VEGF reduces ischemic damage via ERK-1/2 dependent pathways [11, 13]. Surprisingly, in our study employing RIPC-plasma in combination with a cell culture system, VEGF specific ELISAs revealed significantly reduced levels of the protein in protective RIPC plasma. In the first place these data would suggest that VEGF is not involved in RIPC-mediated protection of endothelial cells. However, VEGF influences endothelial cell proliferation and migration and has been reported to stimulate the expression of metalloproteinases (MMPs) in HUVEC cells [4, 70]. We have recently shown that activities of MMP-2 and MMP-9 are reduced by RIPC in cardiac tissue of cardiosurgical patients [73]. These findings suggest that MMPs could be involved in RIPC and VEGF mediated mechanisms, however, further studies are needed to confirm a potential causal relationship between RIPC, VEGF and MMPs.
In conclusion, the results of the present study support the hypothesis that humoral factors confer RIPC-mediated cell and organ protection and we suggest endothelial cells as targets for RIPC-released mediators.
References
Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, Napoli C, Scognamiglio P, Bollella O, Cioppa A, Longobardi G, Rengo F (1997) Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol 30:947–954. doi:10.1016/S0735-1097(97)00256-8
Ahmad AM, Ali GS, Tariq W (2014) Remote ischemic preconditioning is a safe adjuvant technique to myocardial protection but adds no clinical benefit after on-pump coronary artery bypass grafting. Heart Surg Forum 17:E220–E223. doi:10.1532/HSF98.2014391
Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, Schuett T, Lauer F, Maahs D, Hummitzsch L, Cremer J, Zacharowski K, Meybohm P (2013) Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol 108:314. doi:10.1007/s00395-012-0314-0
Bernatchez PN, Soker S, Sirois MG (1999) Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 274:31047–31054. doi:10.1074/jbc.274.43.31047
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. doi:10.1016/S0140-6736(09)62001-8
Bromage DI, Davidson SM, Yellon DM (2014) Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther 143:305–315. doi:10.1016/j.pharmthera.2014.03.009
Cai Z, Luo W, Zhan H, Semenza GL (2013) Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA 110:17462–17467. doi:10.1016/j.pharmthera.2014.03.009
Cai ZP, Parajuli N, Zheng X, Becker L (2012) Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol 107:277. doi:10.1007/s00395-012-0277-1
Candilio L, Malik A, Ariti C, Barnard M, Di SC, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM (2014) Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart 101(3):185–192. doi:10.1136/heartjnl-2014-306178
Carrasco-Chinchilla F, Munoz-Garcia AJ, Dominguez-Franco A, Millan-Vazquez G, Guerrero-Molina A, Ortiz-Garcia C, Enguix-Armada A, Alonso-Briales JH, Hernandez-Garcia JM, de Teresa-Galvan E, Jimenez-Navarro MF (2013) Remote ischaemic postconditioning: does it protect against ischaemic damage in percutaneous coronary revascularisation? Randomised placebo-controlled clinical trial. Heart 99:1431–1437. doi:10.1136/heartjnl-2013-304172
Celec P, Yonemitsu Y (2004) Vascular endothelial growth factor—basic science and its clinical implications. Pathophysiology 11:69–75. doi:10.1016/j.pathophys.2004.03.002
Cohen MV, Downey JM (2014) Signaling pathways and mechanisms of protection in pre- and postconditioning: historical perspective and lessons for the future. Br J Pharmacol. doi:10.1111/bph.12903
Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA (2014) Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. doi:10.1016/j.cyto.2014.08.005
Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP (2013) Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv 6:246–251. doi:10.1161/CIRCINTERVENTIONS.112.000184
Ebrahim Z, Yellon DM, Baxter GF (2007) Ischemic preconditioning is lost in aging hypertensive rat heart: independent effects of aging and longstanding hypertension. Exp Gerontol 42:807–814. doi:10.1016/j.exger.2007.04.005
Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R (2014) Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66:1142–1174. doi:10.1016/j.exger.2007.04.005
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59:418–458. doi:10.1124/pr.107.06002
Gendaszewska-Darmach E, Maszewska M, Zaklos M, Koziolkiewicz M (2003) Degradation of extracellular nucleotides and their analogs in HeLa and HUVEC cell cultures. Acta Biochim Pol 50:973–984 (035004973)
Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P (2014) Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 68:75–78. doi:10.1016/j.yjmcc.2014.01.004
Hartel FV, Holl M, Arshad M, Aslam M, Gunduz D, Weyand M, Micoogullari M, Abdallah Y, Piper HM, Noll T (2010) Transient hypoxia induces ERK-dependent anti-apoptotic cell survival in endothelial cells. Am J Physiol Cell Physiol 298:C1501–C1509. doi:10.1152/ajpcell.00333.2009
Hausenloy DJ (2013) Cardioprotection techniques: preconditioning, postconditioning and remote conditioning (basic science). Curr Pharm Des 19:4544–4563. doi:10.2174/1381612811319250004
Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, Evans R, Robertson S, Knight R, Ariti C, Clayton T, Yellon DM (2012) Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol 101:339–348. doi:10.1007/s00392-011-0397-x
Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, Kremastinos DT, Yellon DM (2012) Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther 26:87–93. doi:10.1007/s10557-011-6364-y
Hausenloy DJ, Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79:377–386. doi:10.1093/cvr/cvn114
Heusch G (2012) HIF-1alpha and paradoxical phenomena in cardioprotection. Cardiovasc Res 96:214–215. doi:10.1093/cvr/cvs145
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699. doi:10.1161/CIRCRESAHA.116.305348
Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. doi:10.1016/j.jacc.2014.10.031
Heusch G, Kleinbongard P, Skyschally A, Levkau B, Schulz R, Erbel R (2012) The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc Res 94:237–245. doi:10.1093/cvr/cvr271
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. doi:10.1016/S0140-6736(14)60107-0
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M (2012) STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 110:111–115. doi:10.1161/CIRCRESAHA.111.259556
Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F, Prunier F (2013) Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS One 8:e77211. doi:10.1371/journal.pone.0077211
Hieber S, Huhn R, Hollmann MW, Weber NC, Preckel B (2009) Hypoxia-inducible factor 1 and related gene products in anaesthetic-induced preconditioning. Eur J Anaesthesiol 26:201–206. doi:10.1097/EJA.0b013e3283212cbb
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, Dutka DP (2009) Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation 119:820–827. doi:10.1161/CIRCULATIONAHA.108.809723
Huffmyer J, Raphael J (2009) Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth 13:5–18. doi:10.1177/1089253208330709
Hummitzsch L, Zitta K, Bein B, Steinfath M, Albrecht M (2014) Culture media from hypoxia conditioned endothelial cells protect human intestinal cells from hypoxia/reoxygenation injury. Exp Cell Res 322:62–70. doi:10.1016/j.yexcr.2013.12.022
Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, Karavolias G, Kremastinos DT (2006) Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart 92:1821–1826. doi:10.1136/hrt.2006.089060
Kageyama S, Hata K, Tanaka H, Hirao H, Kubota T, Okamura Y, Iwaisako K, Takada Y, Uemoto S (2014) Intestinal Ischemic preconditioning ameliorates hepatic ischemia reperfusion injury in rats: role of heme oxygenase-1 in the second-window of protection. Liver Transpl 21(1):112–122. doi:10.1002/lt.24006
Kalakech H, Hibert P, Prunier-Mirebeau D, Tamareille S, Letournel F, Macchi L, Pinet F, Furber A, Prunier F (2014) RISK and SAFE Signaling pathway involvement in apolipoprotein A-I-induced cardioprotection. PLoS One 9:e107950. doi:10.1371/journal.pone.0107950
Kalakech H, Tamareille S, Pons S, Godin-Ribuot D, Carmeliet P, Furber A, Martin V, Berdeaux A, Ghaleh B, Prunier F (2013) Role of hypoxia inducible factor-1alpha in remote limb ischemic preconditioning. J Mol Cell Cardiol 65:98–104. doi:10.1016/j.yjmcc.2013.10.001
Kleinbongard P, Heusch G (2014) Extracellular signalling molecules in the ischaemic/reperfused heart—druggable and translatable for cardioprotection? Br J Pharmacol. doi:10.1111/bph.12902
Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19:143–150. doi:10.1152/physiolgenomics.00046.2004
Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M (1993) Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res 72:1293–1299. doi:10.1161/01.RES.72.6.1293
Lavi S, Lavi R (2011) Conditioning of the heart: from pharmacological interventions to local and remote protection: possible implications for clinical practice. Int J Cardiol 146:311–318. doi:10.1016/j.ijcard.2010.08.010
Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN (2014) MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109:423. doi:10.1007/s00395-014-0423-z
Li SJ, Wu YN, Kang Y, Yin YQ, Gao WZ, Liu YX, Lou JS (2010) Noninvasive limb ischemic preconditioning protects against myocardial I/R injury in rats. J Surg Res 164:162–168. doi:10.1016/j.jss.2009.03.017
Lim SY, Yellon DM, Hausenloy DJ (2010) The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105:651–655. doi:10.1007/s00395-010-0099-y
Manchurov V, Ryazankina N, Khmara T, Skrypnik D, Reztsov R, Vasilieva E, Shpektor A (2014) Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am J Med 127:670–673. doi:10.1016/j.amjmed.2014.02.012
McDonald MA, Braga JR, Li J, Manlhiot C, Ross HJ, Redington AN (2014) A randomized pilot trial of remote ischemic preconditioning in heart failure with reduced ejection fraction. PLoS One 9:e105361. doi:10.1371/journal.pone.0105361
Meybohm P, Zacharowski K, Cremer J, Roesner J, Kletzin F, Schaelte G, Felzen M, Strouhal U, Reyher C, Heringlake M, Schon J, Brandes I, Bauer M, Knuefermann P, Wittmann M, Hachenberg T, Schilling T, Smul T, Maisch S, Sander M, Moormann T, Boening A, Weigand MA, Laufenberg R, Werner C, Winterhalter M, Treschan T, Stehr SN, Reinhart K, Hasenclever D, Brosteanu O, Bein B (2012) Remote ischaemic preconditioning for heart surgery. The study design for a multi-center randomized double-blinded controlled clinical trial—the RIPHeart-Study. Eur Heart J 33:1423–1426. doi:10.1093/eurheartj/ehs131
Michiels C (2003) Endothelial cell functions. J Cell Physiol 196:430–443. doi:10.1002/jcp.10333
Minamino T (2012) Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J 76:1074–1082. doi:10.1253/circj.CJ-12-0132 (0022-3565/00/2941-0263)
Moro L, Pedone C, Mondi A, Nunziata E, Inc Antonelli (2011) Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis 219:750–752. doi:10.1016/j.atherosclerosis.2011.08.046
Murphey LJ, Hachey DL, Oates JA, Morrow JD, Brown NJ (2000) Metabolism of bradykinin in vivo in humans: identification of BK1-5 as a stable plasma peptide metabolite. J Pharmacol Exp Ther 294:263–269 (0022-3565/00/2941-0263)
Oberkofler CE, Limani P, Jang JH, Rickenbacher A, Lehmann K, Raptis DA, Ungethuem U, Tian Y, Grabliauskaite K, Humar R, Graf R, Humar B, Clavien PA (2014) Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology 60:1409–1417. doi:10.1002/hep.27089
Pitcher JM, Wang M, Tsai BM, Kher A, Turrentine MW, Brown JW, Meldrum DR (2005) Preconditioning: gender effects. J Surg Res 129:202–220. doi:10.1002/hep.27089
Prasad A, Gossl M, Hoyt J, Lennon RJ, Polk L, Simari R, Holmes DR Jr, Rihal CS, Lerman A (2013) Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv 81:930–936. doi:10.1002/ccd.24443
Przyklenk K, Whittaker P (2011) Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther 16:255–259. doi:10.1177/1074248411409040
Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS (2010) Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122:S53–S59. doi:10.1161/CIRCULATIONAHA.109.926667
Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M (2014) Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114:1601–1610. doi:10.1161/CIRCRESAHA.114.303822
Sabbagh S, Henry Salzman MM, Kloner RA, Simkhovich BZ, Rezkalla SH (2013) Remote ischemic preconditioning for coronary artery bypass graft operations. Ann Thorac Surg 96:727–736. doi:10.1016/j.athoracsur.2013.02.049
Sanada S, Komuro I, Kitakaze M (2011) Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 301:H1723–H1741. doi:10.1152/ajpheart.00553.2011
Seal JB, Gewertz BL (2005) Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 19:572–584. doi:10.1007/s10016-005-4616-7
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408. doi:10.1016/j.cell.2012.01.021
Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE (2014) Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35:168–175. doi:10.1093/eurheartj/eht369
Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:H1598–H1603. doi:10.1152/ajpheart.00396.2010
Surendra H, Diaz RJ, Harvey K, Tropak M, Callahan J, Hinek A, Hossain T, Redington A, Wilson GJ (2013) Interaction of delta and kappa opioid receptors with adenosine A1 receptors mediates cardioprotection by remote ischemic preconditioning. J Mol Cell Cardiol 60:142–150. doi:10.1016/j.yjmcc.2013.04.010
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105:657–664. doi:10.1007/s00395-010-0104-5
Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G (2013) Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382:597–604. doi:10.1016/S0140-6736(13)61450-6
Unemori EN, Ferrara N, Bauer EA, Amento EP (1992) Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol 153:557–562. doi:10.1002/jcp.1041530317
Weber NC, Kandler J, Schlack W, Grueber Y, Fradorf J, Preckel B (2008) Intermitted pharmacologic pretreatment by xenon, isoflurane, nitrous oxide, and the opioid morphine prevents tumor necrosis factor alpha-induced adhesion molecule expression in human umbilical vein endothelial cells. Anesthesiology 108:199–207. doi:10.1097/01.anes.0000299441.32091.ed
Xia Y, Choi HK, Lee K (2012) Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem 49:24–40. doi:10.1016/j.ejmech.2012.01.033
Zitta K, Meybohm P, Bein B, Gruenewald M, Lauer F, Steinfath M, Cremer J, Zacharowski K, Albrecht M (2014) Activities of cardiac tissue matrix metalloproteinases 2 and 9 are reduced by remote ischemic preconditioning in cardiosurgical patients with cardiopulmonary bypass. J Transl Med 12:94. doi:10.1186/1479-5876-12-94
Zitta K, Meybohm P, Bein B, Heinrich C, Renner J, Cremer J, Steinfath M, Scholz J, Albrecht M (2012) Serum from patients undergoing remote ischemic preconditioning protects cultured human intestinal cells from hypoxia-induced damage: involvement of matrixmetalloproteinase-2 and -9. Mol Med 18:29–37. doi:10.2119/molmed.2011.00278
Zitta K, Meybohm P, Bein B, Huang Y, Heinrich C, Scholz J, Steinfath M, Albrecht M (2012) Salicylic acid induces apoptosis in colon carcinoma cells grown in vitro: influence of oxygen and salicylic acid concentration. Exp Cell Res 318:828–834. doi:10.1016/j.yexcr.2012.02.002
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nina C. Weber, Isabelle Riedemann have contributed equally to the present publication.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Weber, N.C., Riedemann, I., Smit, K.F. et al. Plasma from human volunteers subjected to remote ischemic preconditioning protects human endothelial cells from hypoxia–induced cell damage. Basic Res Cardiol 110, 17 (2015). https://doi.org/10.1007/s00395-015-0474-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0474-9