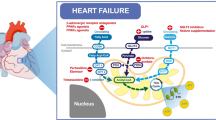
Abstract
Cardiovascular disease is the major cause of death and disability in the world, with ischemic heart disease accounting for the vast majority of this health problem. Current treatments for ischemic heart disease are primarily aimed at either increasing blood and oxygen supply to the heart or decreasing the heart’s oxygen demand. A novel treatment strategy involves increasing the efficiency of oxygen use by the heart. During and following ischemia, the heart can become inefficient in using oxygen, due in part to an excessive use of fatty acids as a source of fuel. One potential strategy to increase cardiac efficiency is to inhibit this use of fatty acid oxidation as a fuel source, while stimulating the use of glucose oxidation as a fuel source, which allows the heart to produce energy more efficiently and reduces the acidosis associated with ischemia/reperfusion, both of which are beneficial to the heart. Malonyl CoA is a potent endogenous inhibitor of cardiac fatty acid oxidation, secondary to inhibition of carnitine palmitoyl transferase-I, the gatekeeper of mitochondrial fatty acid uptake. Malonyl CoA is synthesized in the heart by acetyl CoA carboxylase and degraded by malonyl CoA decarboxylase (MCD). Strategies aimed at increasing cardiac malonyl CoA levels, such as via inhibition of MCD, are associated with a decrease in fatty acid oxidation rates, and a parallel increase in glucose oxidation rates. This is associated with a decrease in acidosis and an improvement in cardiac function and efficiency during and following ischemia. Therefore, targeting malonyl CoA is a novel exciting approach for the treatment of cardiac ischemia/reperfusion.

Similar content being viewed by others
References
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ (2001) Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291:2613–2616
Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC Jr, Anbe DT, Kushner FG, Ornato JP, Pearle DL, Sloan MA, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW (2008) 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 51:210–247
Baron SJ, Li J, Russell RR 3rd, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH (2005) Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res 96:337–345
Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L (2001) Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett 505:348–352
Bianchi A, Evans JL, Iverson AJ, Nordlund AC, Watts TD, Witters LA (1990) Identification of an isozymic form of acetyl-CoA carboxylase. J Biol Chem 265:1502–1509
Campbell FM, Kozak R, Wagner A, Altarejos JY, Dyck JR, Belke DD, Severson DL, Kelly DP, Lopaschuk GD (2002) A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem 277:4098–4103
Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI (2007) Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA 104:16480–16485
Cuthbert KD, Dyck JR (2005) Malonyl-CoA decarboxylase is a major regulator of myocardial fatty acid oxidation. Curr Hypertens Rep 7:407–411
Dennis SC, Gevers W, Opie LH (1991) Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol 23:1077–1086
Diaz R, Goyal A, Mehta SR, Afzal R, Xavier D, Pais P, Chrolavicius S, Zhu J, Kazmi K, Liu L, Budaj A, Zubaid M, Avezum A, Ruda M, Yusuf S (2007) Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA 298:2399–2405
Dyck JR (2007) The ischemic heart: starving to stimulate the adiponectin-AMPK signaling axis. Circulation 116:2779–2781
Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD (1998) Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol 275:H2122–H2129
Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, Nadzan AM, Lopaschuk GD (2004) Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 94:e78–e84
Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD (2006) Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation 114:1721–1728
Dyck JR, Lopaschuk GD (2006) AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol 574:95–112
Dyck JR, Lopaschuk GD (2002) Malonyl CoA control of fatty acid oxidation in the ischemic heart. J Mol Cell Cardiol 34:1099–1109
Essop MF, Camp HS, Choi CS, Sharma S, Fryer RM, Reinhart GA, Guthrie PH, Bentebibel A, Gu Z, Shulman GI, Taegtmeyer H, Wakil SJ, Abu-Elheiga L (2008) Reduced heart size and increased myocardial fuel substrate oxidation in ACC2 mutant mice. Am J Physiol Heart Circ Physiol 295:H256–H265
Fath-Ordoubadi F, Beatt KJ (1997) Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation 96:1152–1156
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP (2002) The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109:121–130
Folmes CD, Clanachan AS, Lopaschuk GD (2006) Fatty acids attenuate insulin regulation of 5′-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ Res 99:61–68
Folmes CD, Lopaschuk GD (2007) Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res 73:278–287
Folmes CDL, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD (2008) Suppression of AMP-Activated Protein Kinase Activity Does Not Impair Recovery of Contractile Function During Reperfusion of Ischemic Hearts. Submitted
Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB (2003) Years of life lost due to obesity. Jama 289:187–193
Hubert HB, Feinleib M, McNamara PM, Castelli WP (1983) Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67:968–977
Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS (2002) Obesity and the risk of heart failure. N Engl J Med 347:305–313
Kewalramani G, An D, Kim MS, Ghosh S, Qi D, Abrahani A, Pulinilkunnil T, Sharma V, Wambolt RB, Allard MF, Innis SM, Rodrigues B (2007) AMPK control of myocardial fatty acid metabolism fluctuates with the intensity of insulin-deficient diabetes. J Mol Cell Cardiol 42:333–342
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete Fatty Acid oxidation contribute to skeletal muscle insulin resistance. Cell metabolism 7:45–56
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD (1995) High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270:17513–17520
Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD (1996) Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta 1301:67–75
Kurien VA, Oliver MF (1971) Free fatty acids during acute myocardial infarction. Prog Cardiovasc Dis 13:361–373
Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS (2004) Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J 378:983–990
Liu B, Clanachan AS, Schulz R, Lopaschuk GD (1996) Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res 79:940–948
Liu B, el Alaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD (1996) Uncoupling of contractile function from mitochondrial TCA cycle activity and MVO2 during reperfusion of ischemic hearts. Am J Physiol 270:H72–H80
Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD (2002) High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 39:718–725
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO (1994) Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213:263–276
Lopaschuk GD, Collins-Nakai R, Olley PM, Montague TJ, McNeil G, Gayle M, Penkoske P, Finegan BA (1994) Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J 128:61–67
Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, Allard MF (2002) Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res 53:841–851
Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ (2006) Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA 103:8552–8557
Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L (2005) Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 293:437–446
Mueller HS, Ayres SM (1978) Metabolic response of the heart in acute myocardial infarction in man. Am J Cardiol 42:363–371
Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, Chirala SS, Wakil SJ (2005) Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci USA 102:1384–1389
Petersen KF, Shulman GI (2006) Etiology of insulin resistance. Am J Med 119:S10–S16
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Reszko AE, Kasumov T, Comte B, Pierce BA, David F, Bederman IR, Deutsch J, Des Rosiers C, Brunengraber H (2001) Assay of the concentration and 13C-isotopic enrichment of malonyl-coenzyme A by gas chromatography-mass spectrometry. Anal Biochem 298:69–75
Russell R 3rd (2006) Stress signaling in the heart by AMP-activated protein kinase. Curr Hypertens Rep 8:446–450
Russell RR 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH (2004) AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114:495–503
Saddik M, Gamble J, Witters LA, Lopaschuk GD (1993) Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 268:25836–25845
Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB (2000) Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta -D-ribofuranoside. J Biol Chem 275:24279–24283
Sambandam N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD (2006) Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol 290:H87–H95
Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K (2005) Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11:1096–1103
Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R (2007) Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 116:2809–2817
Sim AT, Hardie DG (1988) The low activity of acetyl-CoA carboxylase in basal and glucagon-stimulated hepatocytes is due to phosphorylation by the AMP-activated protein kinase and not cyclic AMP-dependent protein kinase. FEBS Lett 233:294–298
Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, Lopaschuk GD (2005) Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol Heart Circ Physiol 289:H2304–H2309
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129
Tani M, Neely JR (1990) Na+ accumulation increases Ca2+ overload and impairs function in anoxic rat heart. J Mol Cell Cardiol 22:57–72
Tani M, Neely JR (1989) Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res 65:1045–1056
Thampy KG (1989) Formation of malonyl coenzyme A in rat heart. Identification and purification of an isozyme of A carboxylase from rat heart. J Biol Chem 264:17631–17634
Timmer JR, van der Horst IC, Ottervanger JP, De Luca G, van ‘t Hof AW, Bilo HJ, Zijlstra F (2004) Glucose-insulin-potassium infusion as adjunctive therapy in myocardial infarction: current evidence and potential mechanisms. Ital Heart J 5:727–731
Ussher JR, Lopaschuk GD (2006) Clinical implications of energetic problems in cardiovascular disease. Heart Metab 32:9–17
Ussher JR, Lopaschuk GD (2008) The malonyl CoA axis as a potential target for treating ischaemic heart disease. Cardiovasc Res 79:259–268
Wang W, Ussher JER, Wang S, Dyck JRB, Lopaschuk GD (2007) Malonyl CoA decarboxylase deficient mice display minimal infarct during in vivo ischemia/reperfusion. J Mol Cell Cardiol 42:S194–S195
Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR (1978) Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem 253:4305–4309
Young ME, Goodwin GW, Ying J, Guthrie P, Wilson CR, Laws FA, Taegtmeyer H (2001) Regulation of cardiac and skeletal muscle malonyl-CoA decarboxylase by fatty acids. Am J Physiol Endocrinol Metab 280:E471–E479
Yusuf S, Mehta SR, Diaz R, Paolasso E, Pais P, Xavier D, Xie C, Ahmed RJ, Khazmi K, Zhu J, Liu L (2004) Challenges in the conduct of large simple trials of important generic questions in resource-poor settings: the CREATE and ECLA trial program evaluating GIK (glucose, insulin and potassium) and low-molecular-weight heparin in acute myocardial infarction. Am Heart J 148:1068–1078
Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L (2006) Role of the alpha2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol 291:H2875–H2883
Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI (2007) Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104:17075–17080
NHLBI morbidity and mortality chart-book (2004) National Heart, Lung, and Blood Institute, Bethesda, May 2004 (Accessed October 23, 2008, at http://www.nhlbi.nih.gov/resources/docs/cht-book.htm)
Acknowledgments
Supported by a grant from the Canadian Institutes for Health Research Grant to GDL. JRU is a trainee of the Alberta Heritage Foundation for Medical Research and Tomorrow’s Research Cardiovascular Health Professionals (TORCH). GDL is a Medical Scientist of the Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ussher, J.R., Lopaschuk, G.D. Targeting malonyl CoA inhibition of mitochondrial fatty acid uptake as an approach to treat cardiac ischemia/reperfusion. Basic Res Cardiol 104, 203–210 (2009). https://doi.org/10.1007/s00395-009-0003-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0003-9