Abstract
Purpose
Iron absorption in sickle cell anemia (SCA) remains unclear and studies in adults with SCA are scarce. The aim of this study was to evaluate the iron absorption SCA adults and its association with iron status and hepcidin concentration.
Methods
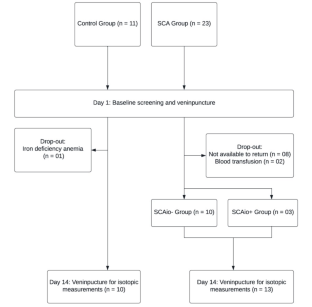
SCA patients (n = 13; SCAtotal) and control participants (n = 10) ingested an oral stable iron isotope (57Fe). Iron absorption was measured by inductively coupled plasma mass spectrometry (ICP-MS) 14 days after isotope administration. Patients with ≥ 1000 ng/mL serum ferritin were considered to present iron overload (IO) (SCAio+; n = 3) and others classified without IO (SCAio-; n = 10).
Results
Iron absorption in the control group ranged from 0.3 to 26.5% (median = 0.9%), while it varied from 0.3 to 5.4% in SCAio+ (median = 0.5%) and from 0.3 to 64.2% in the SCAio- (median = 6.9%). Hepcidin median values were 14.1 ng/mL (3.0–31.9 ng/mL) in SCAio-, 6.2 ng/mL (3.3–7.8 ng/mL) in SCAio + and 6.2 ng/mL (0.6–9.3 ng/mL) in control. Iron absorption was associated with ferritin level (r = − 0.641; p = 0.018) and liver iron concentration (LIC; r = − 0.786; p = 0.036) in the SCAtotal group.
Conclusion
Our data suggest that SCAio- individuals may be at risk of developing primary IO. Simultaneously, secondary IO may induce physiological adaptation, resulting in reduced iron absorption. Further studies evaluating intestinal iron absorption using larger sample sizes should be conducted to help establish a safe nutrition approach to be adopted and to ensure the security of food-fortifying public policies for these patients.
Trial registration
This trial was registered at www.ensaiosclinicos.gov.br (Identifier RBR-4b7v8pt).


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Conran N, Franco-Penteado CF, Costa FF (2009) Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin 33(1):1–16. https://doi.org/10.1080/03630260802625709
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376(9757):2018–2031. https://doi.org/10.1016/S0140-6736(10)61029-X
Modell B, Darlinson M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86(6):480–487. https://doi.org/10.2471/blt.06.036673
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L et al (2018) Sickle cell disease. Nat Rev Dis Primers 4:18010. https://doi.org/10.1038/nrdp.2018.10
Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T et al (2000) Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 96(1):76–79. https://doi.org/10.1182/blood.V96.1.76
Josephson CD, Su LL, Hillyer KL, Hillyer CD (2007) Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev 21(2):118–133. https://doi.org/10.1016/j.tmrv.2006.11.003
Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339(1):5–11. https://doi.org/10.1056/NEJM199807023390102
Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators (2005) Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med 353(26):2769–2778. https://doi.org/10.1056/NEJMoa050460
Inati A, Khoriaty E, Mussalam KM (2011) Iron sickle-cell disease: what have we learned over the years? Pediatr Blood Cancer 56(2):182–190. https://doi.org/10.1002/pbc.22721
Thuret I (2013) Post-transfusional iron overload in the haemoglobinopathies. C R Biol 336(3):164–172. https://doi.org/10.1016/j.crvi.2012.09.010
Cohen A, Masera G, Zoumbos N, Uysal Z, Boulet D, Watman N et al (2005) Effect of iron intake on control of body iron in patients with Thalassemia major treated with Deferasirox (Exjade®, ICL670). Blood 106(11):822. https://doi.org/10.1182/blood.V106.11.822.822
Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Capellini MD et al (2008) Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol 80(2):168–176. https://doi.org/10.1111/j.1600-0609.2007.00985.x
Hoffbrand AV, Taher A, Cappellini MD (2012) How I treat transfusional iron overload. Blood 120(18):3657–3669. https://doi.org/10.1182/blood-2012-05-370098
Ward R (2010) An update on disordered iron metabolism and iron overload. Hematology 15(5):311–317. https://doi.org/10.1179/102453310X12647083621164
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta Mol Cell Res 1823(9):1434–1443. https://doi.org/10.1016/j.bbamcr.2012.01.014
Muckenthaler MU, Rivella S, Hentze MW, Galy B (2017) A Red Carpet for Iron Metabolism. Cell 168(3):344–361. https://doi.org/10.1016/j.cell.2016.12.034
Ganz T, Nemeth E (2006) Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290(2):G199–203. https://doi.org/10.1152/ajpgi.00412.2005
Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ (2018) Biomarkers of Nutrition for Development (BOND)-Iron review. J Nutr 148(suppl1):1001S–1067S. https://doi.org/10.1093/jn/nxx036
Nemeth E (2008) Iron regulation and erythropoiesis. Curr Opin Hematol 15(3):169–175. https://doi.org/10.1097/MOH.0b013e3282f73335
Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101(7):2461–2463. https://doi.org/10.1182/blood-2002-10-3235
Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105(2):260–272. https://doi.org/10.3324/haematol.2019.232124
Omena J, Cople-Rodrigues CS, Cardoso JDA, Soares AR, Fleury MK, Brito FSB et al (2018) Serum hepcidin concentration in individuals with sickle cell anemia: basis for the dietary recommendation of iron. Nutrients 10(4):498. https://doi.org/10.3390/nu10040498
Mangaonkar AA, Thawer F, Son J, Ajebo G, Xu H, Barrett NJ et al (2020) Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br J Haematol 189(6):1204–1209. https://doi.org/10.1111/bjh.16498
Ringelhann B, Konotey-Ahulu F, Dodu SR (1970) Studies on iron metabolism in sickle cell anaemia, sickle cell haemoglobin C disease, and haemoglobin C disease using a large volume liquid scintillation counter. J Clin Pathol 23(2):127–134. https://doi.org/10.1136/jcp.23.2.127
Borges R, Mancuso A, Camey S, Leotti V, Hirakata V, Azambuja G, Castro S (2021) Power and Sample Size for Health Researchers a tool for calculating sample size and statistical power designed for health researchers. Clin Biomed Res 40(4). https://doi.org/10.22491/2357-9730.109542
Porter J, Garbowski M (2013) Consequences and management of iron overload in sickle cell disease. Hematol Am Soc Hematol Educ Program 2013:447–456. https://doi.org/10.1182/asheducation-2013.1.447
Walczyk T, von Blanckenburg F (2002) Natural iron isotope variations in human blood. Science 295(5562):2065–2066. https://doi.org/10.1126/science.1069389
Abrams SA (1999) Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 70(6):955–964. https://doi.org/10.1093/ajcn/70.6.955
van den Heuvel EG, Muys T, Pellegrom H, Bruyntjes JP, van Dokkum W, Spanhaak S, Schaafsma G (1998) A new method to measure iron absorption from the enrichment of 57Fe and 58Fe in young erythroid cells. Clin Chem 44(3):649–654
Fidler MC, Davidsson L, Zeder C, Walczyk T, Hurrell RF (2003) Iron absorption from ferrous fumarate in adult women is influenced by ascorbic acid but not by Na2EDTA. Br J Nutr 90(6):1081–1085. https://doi.org/10.1079/bjn2003995
Junqueira-Franco MVM, Dutra de Oliveira JE, Nutti MR, Pereira HS, Carvalho JLV, Abrams SA et al (2018) Iron absorption from beans with different contents of iron, evaluated by stable isotopes. Clin Nutr ESPEN 25:121–125. https://doi.org/10.1016/j.clnesp.2018.03.120
International Atomic Energy Agency (IAEA) (2012) Assessment of iron bioavailability in humans using stable iron isotope techniques. Vienna: International Atomic Energy Agency 77 p. No.: 21
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF (1994) A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 7(3):411–424. https://doi.org/10.1079/bjn19940148
Chen Z, Griffin IJ, Plumlee LM, Abrams SA (2005) High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr 135(7):1790–1795. https://doi.org/10.1093/jn/135.7.1790
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C et al (2000) Hepatic iron concentration and total body iron stores in Thalassemia major. N Engl J Med 343(5):327–331. https://doi.org/10.1056/NEJM200008033430503
St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105(2):855–861. https://doi.org/10.1182/blood-2004-01-0177
Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113(20):4853–4855. https://doi.org/10.1182/blood-2008-12-191643
Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D et al (2018) Liver iron quantification with MR imaging: a primer for radiologists. Radiographics 38(2):392–412. https://doi.org/10.1148/rg.2018170079
Institute of Medicine (US) Panel on Micronutrients (2001) Dietary reference intakes for. In: Vitamin A, Vitamin K (eds) Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US)
Teixeira TV, Da Silva ACF, Rodrigues CDSC, Brito FDSB, Canella DS, Citelli M (2023) Food Consumption of people with Sickle Cell Anemia in a Middle-Income Country. Nutrients15(6):1478. https://doi.org/10.3390/nu15061478
Li H, Kazmi JS, Lee S, Zhang D, Gao X, Maryanovich M et al (2023) Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease. Blood 141(2):194–199. https://doi.org/10.1182/blood.2022016218
Sarria B, Dainty JR (2010) Comparison of faecal monitoring and area under the curve techniques to determine iron absorption in humans using stable isotope labelling. J Trace Elem Med Biol 24(3):157–160. https://doi.org/10.1016/j.jtemb.2010.01.010
Sudarev VV, Dolotova SM, Bukhalovich SM, Bazhenov SV, Ryzhykau YL, Uversky VN et al (2023) Ferritin self-assembly, structure, function, and biotechnological applications. Int J Biol Macromol 224:319–343. https://doi.org/10.1016/j.ijbiomac.2022.10.126
Cook JD, Lipschitz DA, Miles LEM, Finch CA (1974) Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr 27(7):681–687. https://doi.org/10.1093/ajcn/27.7.681
Lynch SR, Skikne BS, Cook JD (1989) Food iron absorption in idiopathic hemochromatosis. Blood 74(6):2187–2193
Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ (2009) Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr 89(4):1088–1091. https://doi.org/10.3945/ajcn.2008.27297
Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF (2009) Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr 90(5):1280–1287. https://doi.org/10.3945/ajcn.2009.28129
Erlandson ME, Walden B, Stern G, Hilgartner MW, Wehman J, Smith CH (1962) Studies on congenital hemolytic syndromes, IV. Gastrointestinal absorption of iron. Blood 19:359–378
Acknowledgements
The authors express their gratitude to the volunteers who participated in this study. They would also like to acknowledge the excellent technical assistance provided by Isis Rodrigues, Viviane F.Meneses, Clarice M. Carvalho, Elizabeth Pereira, Verônica Barbosa, and Valdilene L. Souza.
Funding
This study was supported by the Ministry of Health (process # 777022/2012); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process # 408401/2017-6); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (process # E-26-010.100930/2018 and E-26/200.963/2022).
Author information
Authors and Affiliations
Contributions
MC, JO, FFB, and CSCR designed the research; JO, MC, and VMV conducted the research; JO, VMV, MC, BFB and RES conducted the laboratory analysis; FFB and CMD helped to interpret the data and provided critical suggestions and comments; GFJ and ASR conducted the MRI procedures; JO, FFB and MC performed the statistical analysis, wrote the manuscript, and had primary responsibility for the final content. All authors read, contributed and approved the final manuscript. None of the authors declared any personal or financial conflict of interest.
Corresponding authors
Ethics declarations
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki principles. The Ethical Committee of Hemorio (419/17; 2.788.659) and Pedro Ernesto University Hospital (2.695.418) approved the study protocol. Written informed consent was obtained from each participant.
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omena¹, J., Bezerra¹, F.F., Voll¹, V.M. et al. Iron absorption in adults with sickle cell anemia: a stable-isotope approach. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03417-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03417-8




