Abstract
Purpose
Oxidative stress has been reported to cause telomere attrition, which triggers cell apoptosis. Apoptosis of neurocytes may play an essential role in the pathogenesis of neurodegenerative diseases. This study hypothesized that folic acid (FA) supplementation decreased neurocyte apoptosis by alleviating oxidative stress-induced telomere attrition in 25-month-old Sprague Dawley (SD) rats.
Methods
Three-month-old male SD rats were randomly divided into four diet groups by different concentrations of folic acid in equal numbers, with intervention for 22 months. Folate, homocysteine (Hcy), reactive oxygen species (ROS) levels, antioxidant activities, and telomere length in the brain tissues were tested at 11, 18, and 22 months of intervention, and 8-hydroxy-deoxyguanosine (8-OHdG) levels, neurocyte apoptosis and telomere length in the cerebral cortex and hippocampal regions were tested during the 22-month intervention. An automated chemiluminescence system, auto-chemistry analyzer, Q-FISH, qPCR, and TUNEL assay were used in this study.
Results
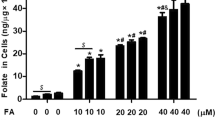
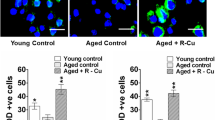
The rats had lower folate concentrations and higher Hcy, ROS, and 8-OHdG concentrations in brain tissue with aging. However, FA supplementation increased folate concentrations and antioxidant activities while decreasing Hcy, ROS, and 8-OHdG levels in rat brain tissue after 11, 18, and 22 months of intervention. Furthermore, FA supplementation alleviated telomere length shortening and inhibited neurocyte apoptosis during the 22-month intervention.
Conclusion
FA supplementation alleviated oxidative stress-induced telomere attrition and inhibited apoptosis of neurocytes in 25-month-old rats.







Similar content being viewed by others
Data availability
The data supporting this study's findings are available on request from the corresponding author upon reasonable request.
References
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783. https://doi.org/10.1126/science.aag2590
Hou Y, Dan X, Babbar M et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15(10):565–581. https://doi.org/10.1038/s41582-019-0244-7
Droge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6(3):361–370. https://doi.org/10.1111/j.1474-9726.2007.00294.x
Gong Z, Huang J, Xu B et al (2019) Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflammation 16(1):62. https://doi.org/10.1186/s12974-019-1450-3
Aguado J, d’Adda di Fagagna F, Wolvetang E (2020) Telomere transcription in ageing. Ageing Res Rev. https://doi.org/10.1016/j.arr.2020.101115
Sahin E, DePinho RA (2012) Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol 13(6):397–404. https://doi.org/10.1038/nrm3352
Lv X, Wang X, Wang Y et al (2019) Folic acid delays age-related cognitive decline in senescence-accelerated mouse prone 8: alleviating telomere attrition as a potential mechanism. Aging (Albany NY). 11(22):10356–10373. https://doi.org/10.18632/aging.102461
Ionescu-Tucker A, Cotman CW (2021) Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol Aging 107:86–95. https://doi.org/10.1016/j.neurobiolaging.2021.07.014
Bai R, Guo J, Ye XY et al (2022) Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev 77:101619. https://doi.org/10.1016/j.arr.2022.101619
Birben E, Sahiner UM, Sackesen C et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5(1):9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583-019-0132-6
Ao GZ, Chu XJ, Ji YY et al (2014) Antioxidant properties and PC12 cell protective effects of a novel curcumin analogue (2E,6E)-2,6-bis(3,5- dimethoxybenzylidene)cyclohexanone (MCH). Int J Mol Sci 15(3):3970–3988. https://doi.org/10.3390/ijms15033970
Xiao X, Liu J, Hu J et al (2008) Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. Eur J Pharmacol 591(1–3):21–27. https://doi.org/10.1016/j.ejphar.2008.06.045
Tower J (2015) Programmed cell death in aging. Ageing Res Rev 23(Pt A):90–100. https://doi.org/10.1016/j.arr.2015.04.002
Burgos-Barragan G, Wit N, Meiser J et al (2017) Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature 548(7669):549–554. https://doi.org/10.1038/nature23481
Caffrey A, McNulty H, Irwin RE et al (2019) Maternal folate nutrition and offspring health: evidence and current controversies. Proc Nutr Soc 78(2):208–220. https://doi.org/10.1017/S0029665118002689
Gallego-Lopez MDC, Ojeda ML, Romero-Herrera I et al (2022) Folic acid homeostasis and its pathways related to hepatic oxidation in adolescent rats exposed to binge drinking. Antioxidants (Basel). https://doi.org/10.3390/antiox11020362
Mattson MP (2003) Will caloric restriction and folate protect against AD and PD? Neurology 60(4):690–695. https://doi.org/10.1212/01.wnl.0000042785.02850.11
Li W, Ma Y, Li Z et al (2019) Folic acid decreases astrocyte apoptosis by preventing oxidative stress-induced telomere attrition. Int J Mol Sci. https://doi.org/10.3390/ijms21010062
Li Z, Li W, Zhou D et al (2022) Alleviating oxidative damage-induced telomere attrition: a potential mechanism for inhibition by folic acid of apoptosis in neural stem cells. Mol Neurobiol 59(1):590–602. https://doi.org/10.1007/s12035-021-02623-3
Ghasemi A, Jeddi S, Kashfi K (2021) The laboratory rat: Age and body weight matter. EXCLI J 20:1431–1445. https://doi.org/10.17179/excli2021-4072
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951. https://doi.org/10.1093/jn/123.11.1939
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661. https://doi.org/10.1096/fj.07-9574LSF
Lin H, Chen H, Qi B et al (2021) Brain-derived extracellular vesicles mediated coagulopathy, inflammation and apoptosis after sepsis. Thromb Res 207:85–95. https://doi.org/10.1016/j.thromres.2021.09.014
Moore CL, Savenka AV, Basnakian AG (2021) TUNEL assay: a powerful tool for kidney injury evaluation. Int J Mol Sci. https://doi.org/10.3390/ijms22010412
Sharifi-Sanjani M, Meeker AK, Mourkioti F (2017) Evaluation of telomere length in human cardiac tissues using cardiac quantitative FISH. Nat Protoc 12(9):1855–1870. https://doi.org/10.1038/nprot.2017.082
Barkovskaya MS, Bogomolov AG, Knauer NY et al (2017) Development of software and modification of Q-FISH protocol for estimation of individual telomere length in immunopathology. J Bioinform Comput Biol 15(2):1650041. https://doi.org/10.1142/S0219720016500414
Mourkioti F, Kustan J, Kraft P et al (2013) Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy. Nat Cell Biol 15(8):895–904. https://doi.org/10.1038/ncb2790
Shoeb M, Kodali VK, Farris BY et al (2017) Oxidative stress, dna methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact 5:61–69. https://doi.org/10.1016/j.impact.2017.01.001
Zhou D, Sun Y, Qian Z et al (2023) Long-term dietary folic acid supplementation attenuated aging-induced hippocampus atrophy and promoted glucose uptake in 25-month-old rats with cognitive decline. J Nutr Biochem. https://doi.org/10.1016/j.jnutbio.2023.109328
Zhang Y, Ding C, Cai Y et al (2021) Astilbin ameliorates oxidative stress and apoptosis in D-galactose-induced senescence by regulating the PI3K/Akt/m-TOR signaling pathway in the brains of mice. Int Immunopharmacol 99:108035. https://doi.org/10.1016/j.intimp.2021.108035
Chen HI, Ou HC, Chen CY et al (2020) Neuroprotective effect of rhodiola crenulata in d-galactose-induced aging model. Am J Chin Med 48(2):373–390. https://doi.org/10.1142/S0192415X20500196
Chauhan A, Chauhan V (2020) Beneficial effects of walnuts on cognition and brain health. Nutrients. https://doi.org/10.3390/nu12020550
Nigdelioglu Dolanbay S, Kocanci FG, Aslim B (2021) Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed Pharmacother 140:111690. https://doi.org/10.1016/j.biopha.2021.111690
Huang C, Lin Y, Su H et al (2015) Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochem Res 40(1):27–35. https://doi.org/10.1007/s11064-014-1461-5
Shen K, Wang Y, Zhang Y et al (2015) Cocktail of four active components derived from sheng mai san inhibits hydrogen peroxide-induced PC12 cell apoptosis linked with the caspase-3/ROCK1/MLC pathway. Rejuvenation Res 18(6):517–527. https://doi.org/10.1089/rej.2015.1697
Liu J, Head E, Gharib AM et al (2002) Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A 99(4):2356–2361. https://doi.org/10.1073/pnas.261709299
Singh R, Kanwar SS, Sood PK et al (2011) Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol Neurobiol 31(1):83–91. https://doi.org/10.1007/s10571-010-9557-1
Budni J, Zomkowski AD, Engel D et al (2013) Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Exp Neurol 240:112–121. https://doi.org/10.1016/j.expneurol.2012.10.024
Srivastav S, Singh SK, Yadav AK et al (2015) Folic acid supplementation ameliorates oxidative stress, metabolic functions and developmental anomalies in a novel fly model of Parkinson’s Disease. Neurochem Res 40(7):1350–1359. https://doi.org/10.1007/s11064-015-1598-x
Zhang X, Huang Z, Xie Z et al (2020) Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med 160:552–565. https://doi.org/10.1016/j.freeradbiomed.2020.08.029
Longoni A, Bellaver B, Bobermin LD et al (2018) Homocysteine induces glial reactivity in adult rat astrocyte cultures. Mol Neurobiol 55(3):1966–1976. https://doi.org/10.1007/s12035-017-0463-0
Sharma GS, Bhattacharya R, Singh LR (2021) Functional inhibition of redox regulated heme proteins: a novel mechanism towards oxidative stress induced by homocysteine. Redox Biol 46:102080. https://doi.org/10.1016/j.redox.2021.102080
Oskouei Z, Mehri S, Kalalinia F et al (2021) Evaluation of the effect of thymoquinone in d-galactose-induced memory impairments in rats: Role of MAPK, oxidative stress, and neuroinflammation pathways and telomere length. Phytother Res 35(4):2252–2266. https://doi.org/10.1002/ptr.6982
Lei F, Wang W, Fu Y et al (2018) Oxidative stress and mitochondrial dysfunction in parafacial respiratory group induced by maternal cigarette smoke exposure in rat offspring. Free Radic Biol Med 129:169–176. https://doi.org/10.1016/j.freeradbiomed.2018.09.003
Ya BL, Liu Q, Li HF et al (2018) Uric acid protects against focal cerebral ischemia/reperfusion-induced oxidative stress via activating Nrf2 and regulating neurotrophic factor expression. Oxid Med Cell Longev 2018:6069150. https://doi.org/10.1155/2018/6069150
Liu DD, Yuan X, Chu SF et al (2019) CZ-7, a new derivative of Claulansine F, ameliorates 2VO-induced vascular dementia in rats through a Nrf2-mediated antioxidant responses. Acta Pharmacol Sin 40(4):425–440. https://doi.org/10.1038/s41401-018-0078-7
Li X, Liu J, Zhou G et al (2021) BDE-209 and DBDPE induce male reproductive toxicity through telomere-related cell senescence and apoptosis in SD rat. Environ Int 146:106307. https://doi.org/10.1016/j.envint.2020.106307
Kan G, Wang Z, Sheng C et al (2021) Inhibition of DKC1 induces telomere-related senescence and apoptosis in lung adenocarcinoma. J Transl Med 19(1):161. https://doi.org/10.1186/s12967-021-02827-0
Field MS, Stover PJ (2018) Safety of folic acid. Ann N Y Acad Sci 1414(1):59–71. https://doi.org/10.1111/nyas.13499
Wald NJ, Morris JK, Blakemore C (2018) Public health failure in the prevention of neural tube defects: time to abandon the tolerable upper intake level of folate. Public Health Rev 39:2. https://doi.org/10.1186/s40985-018-0079-6
Durga J, van Boxtel MP, Schouten EG et al (2007) Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369(9557):208–216. https://doi.org/10.1016/S0140-6736(07)60109-3
Chen H, Liu S, Ge B et al (2021) Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer’s Disease: a randomized, single-blinded, placebo-controlled trial. J Prev Alzheimers Dis. 8(3):249–256. https://doi.org/10.14283/jpad.2021.22
Zhang Q, Liu J, Duan H et al (2021) Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res 34:43–63. https://doi.org/10.1016/j.jare.2021.06.023
Zhai M, Li B, Duan W et al (2017) Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. https://doi.org/10.1111/jpi.12419
Funding
This research was supported by a grant from the National Natural Science Foundation of China (No. 81730091), Tianjin Research Innovation Project for Postgraduate Students (No. 2021YJSB280).
Author information
Authors and Affiliations
Contributions
Wen Li designed the research, revised the manuscript, and proofread the article as the corresponding author. Dezheng Zhou and Yue Sun completed the experiment, analysis of the data, and drafted the manuscript as the first authors. Cuixia Dong, Zehao Wang, Jing Zhao, and Zhenshu Li completed the rats intervention work together. Guowei Huang designed the research. All authors have read and agreed to the published version of the manuscript. The authors declare no potential conflict of interest.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Compliance with ethical standards
The Tianjin Medical University Animal Ethics Committee approved the experimental protocols of this study (TMUaMEC2019003).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, D., Sun, Y., Dong, C. et al. Folic acid alleviated oxidative stress-induced telomere attrition and inhibited apoptosis of neurocytes in old rats. Eur J Nutr 63, 291–302 (2024). https://doi.org/10.1007/s00394-023-03266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03266-x