Abstract
Purpose
Previously reported associations of protein-rich foods with stroke subtypes have prompted interest in the assessment of individual amino acids. We examined the associations of dietary amino acids with risks of ischaemic and haemorrhagic stroke in the EPIC study.
Methods
We analysed data from 356,142 participants from seven European countries. Dietary intakes of 19 individual amino acids were assessed using validated country-specific dietary questionnaires, calibrated using additional 24-h dietary recalls. Multivariable-adjusted Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of ischaemic and haemorrhagic stroke in relation to the intake of each amino acid. The role of blood pressure as a potential mechanism was assessed in 267,642 (75%) participants.
Results
After a median follow-up of 12.9 years, 4295 participants had an ischaemic stroke and 1375 participants had a haemorrhagic stroke. After correction for multiple testing, a higher intake of proline (as a percent of total protein) was associated with a 12% lower risk of ischaemic stroke (HR per 1 SD higher intake 0.88; 95% CI 0.82, 0.94). The association persisted after mutual adjustment for all other amino acids, systolic and diastolic blood pressure. The inverse associations of isoleucine, leucine, valine, phenylalanine, threonine, tryptophan, glutamic acid, serine and tyrosine with ischaemic stroke were each attenuated with adjustment for proline intake. For haemorrhagic stroke, no statistically significant associations were observed in the continuous analyses after correcting for multiple testing.
Conclusion
Higher proline intake may be associated with a lower risk of ischaemic stroke, independent of other dietary amino acids and blood pressure.
Similar content being viewed by others
Background
While stroke is the second leading cause of death worldwide, the incidence of stroke varies substantially between countries reflecting differences in risk factors including variations in dietary intakes [1]. Differences in dietary protein have been suggested to be important, but previous studies of the associations of dietary protein intake with the risk of stroke have reported conflicting results, possibly reflecting heterogeneity by pathological stroke types and dietary sources of protein [2, 3]. The different sources of dietary protein reflect a different profile of individual dietary amino acids. For example, meat and meat products are the main source of the essential amino acids and glycine, while grain products are the main source of dietary cysteine [4]. A previous prospective study reported an inverse association of dietary cysteine with the risk of total stroke [5], and another reported an inverse association of glutamic acid but a positive association of glycine with stroke mortality [6], but overall there is very limited evidence on the topic. Moreover, it is unclear whether the absolute quantity or the proportions of each individual dietary amino acid (i.e. protein quality) that make up total dietary protein would be more relevant for health. The two main stroke subtypes (ischaemic and haemorrhagic) also have different dietary [7] and non-dietary (including genetic) risk factors [8, 9], which highlights the importance of examining stroke subtypes separately. For example, previous analyses in EPIC (the European Prospective Investigation into Cancer and Nutrition) reported inverse associations of ischaemic stroke with the consumption of fruit and vegetables, dietary fibre and dairy products, while the risk of haemorrhagic stroke was positively associated with egg consumption [7]. Therefore, any associations with dietary amino acids are likely to vary by stroke type.
The aim of the present study was to assess the associations of both absolute and relative quantities of individual dietary amino acids with the risk of stroke subtypes in a prospective study of 356,000 participants from seven European countries. Additionally, we aimed to assess if these associations were independent of blood pressure, the major risk factor for both subtypes of stroke.
Methods
Study population
The present analyses involved 356,142 participants recruited from 20 centres between 1992 and 2000 in seven European countries (Denmark, Germany, Italy, the Netherlands, Spain, Sweden, and the UK), who participated in the EPIC study. Details of the study design have been described previously [10, 11]. The inclusion and exclusion criteria for participants in the present study are shown in Supplementary Fig. 1. All participants provided written informed consent, and the study protocol was approved by the ethical review board of IARC and the institutions where the participants were recruited [10].
Data collection
At recruitment, all participants completed questionnaires on medical history and socio-demographic factors, as well as validated country-specific dietary questionnaires (mostly food frequency questionnaires or diet histories) which asked about diet in the previous year [10]. In addition, a stratified random sample of 8% of participants across all centres also completed a standardised and computerised 24-h recall, on average ~ 1.4 years after recruitment, which was used to calibrate the dietary exposures to reduce between centre heterogeneity and to correct for measurement error [12, 13]. The calibration method and rationale have been described previously [7, 12, 13].
Based on reported dietary intakes in both the baseline dietary questionnaires and 24-h recalls, estimates of individual dietary amino acids were derived by matching to the National Nutrient Database for Standard Reference of the United States (developed at the United States Department of Agriculture, or USDA) food composition tables, and appended to the EPIC Nutrient Database. The matching and validation process has been reported in detail [14]. USDA estimates of dietary amino acids were used because the equivalent estimates were not available from all ten national Food Composition Databases that were originally used in EPIC. Altogether, both calibrated and observed estimates were available for the intakes of 19 individual dietary amino acids. Of the 20 standard amino acids (i.e. amino acids relevant for protein synthesis in humans that may be directly translated from the genetic code), glutamine and asparagine were not available as these two amino acids were affected by deamination reactions during the acid hydrolysis process used to compile the USDA database, resulting in their conversion to glutamic acid and aspartic acid [15, 16]. Cystine (two cysteine molecules joined by a disulfide bond) [17] was estimated instead of cysteine, while hydroxyproline, which is not a standard amino acid, was also estimated. The sums of branched-chain amino acids (isoleucine, leucine, valine), other essential amino acids (histidine, lysine, methionine, phenylalanine, threonine and tryptophan) and non-essential amino acids (alanine, arginine, aspartic acid, cystine, glutamic acid, glycine, hydroxyproline, proline, serine and tyrosine) were also calculated.
The primary outcomes were ischaemic stroke (ICD 9 433-434 or ICD 10 I63) and haemorrhagic stroke (ICD 9 430-431 or ICD 10 I60-I61). In addition, we also considered intracerebral haemorrhage (ICD 9 431 or ICD 10 I61) and subarachnoid haemorrhage (ICD 9 430 or ICD 10 I60) as separate outcomes. For all kinds of stroke, both non-fatal and fatal incident events were considered, and details of the ascertainment process and validation methods have been previously reported [7, 11]. Details on the data collection for blood pressure (available for most of the cohort) and other covariates have also been reported previously [7, 11].
Statistical analyses
Cohort characteristics and dietary intakes of amino acids at baseline were summarised as means (SD), median (25th, 75th percentile) or absolute numbers (%). Spearman correlation coefficients were estimated between the individual dietary amino acids, and also for dietary amino acids with dietary protein and food sources of protein. Intakes of dietary amino acids were expressed in three different ways, primarily as individual amino acids modelled as a percentage of total protein intake (% of total protein), and secondarily as individual amino acids modelled as grams per day (g/day) and grams per 1000 kcal (g/1000 kcal). The three approaches tested the hypotheses that the relative protein quality or protein makeup, the absolute quantity of amino acids, and the relative quantity of amino acids to the overall diet were relevant to the risk of stroke. The primary approach of expressing amino acids as a percentage of total protein was selected since this approach was less susceptible to collinearity with other dietary variables [18]. Because the sum of the individual amino acids did not add up to 100% of total protein (mean = 86.2%, partly because glutamine and asparagine were missing), we included post hoc analyses expressing the amino acids as a percentage of the sum of all available amino acids. The associations of total dietary protein with stroke risk were also assessed, by expressing protein in g/day and g/1000 kcal. Although protein intake is typically expressed relative to body weight in dietary recommendations (g of protein/kg body weight), this approach was not considered here due to previous observations of a strong inverse correlation between this measure and body weight in observational studies, making it unsuitable for epidemiological settings [19].
Using multivariable-adjusted Cox regression, we assessed the hazard ratios (HRs) and 95% confidence intervals (CIs) for sex-specific SD differences in calibrated intakes of each dietary amino acid, and sex-specific SD differences and sex-specific fifths of observed intakes. For each association, a test of the trend was estimated by fitting the calibrated or observed intake of each amino acid as a continuous variable when modelling per SD differences, or by fitting the median values of each fifth as a pseudo-continuous variable when modelling differences by fifths of intake. Tests of non-linearity were performed by using a likelihood-ratio test to compare models with the exposure fitted as a continuous variable versus a categorical variable (fifths of intake), based on the observed intake.
The underlying time variable for the Cox regression was age at recruitment to age at diagnosis, death or administrative censoring. All analyses were stratified by sex and EPIC centre, and adjusted for age at recruitment (continuous, Model 1). Model 2 additionally adjusted for total energy intake (continuous, calibrated or observed intake based on USDA estimates). Model 3 additionally adjusted for smoking status and intensity (never, former, current < 10, 10–19, 20 + cigarettes/day, unknown), current calibrated or observed alcohol consumption (non-drinker (< 0.1), 0.1–4.9, 5.0–14.9, 15–29.9, 30–59.9, 60 + g/day based on USDA estimates), physical activity (inactive, moderately inactive, moderately active, active, unknown), employment status (employed or student, neither employed nor student, unknown) and level of education completed (none or primary, secondary, vocational or university, unknown). Model 4 additionally adjusted for self-reported history of diabetes (yes, no, unknown), prior hypertension (yes, no, unknown) and prior hyperlipidaemia (yes, no, unknown). Model 5 additionally adjusted for body mass index (< 22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, ≥ 30.0 kg/m2, unknown), and was considered the main model in these analyses. As total energy intake was held constant (i.e. adjusted for) in the regression model, a higher intake of any individual amino acid, which contributes to total energy, would result in a simultaneously lower intake of some other energy-providing foods or nutrients [20]. Therefore, in model 6, we removed the adjustment for total energy intake thus allowing the comparison of the results with (Model 5) and without (Model 6) a theoretical substitution effect of the amino acid of interest for an unspecified energy-providing source. To further assess the influence of major macronutrients and to allow the interpretation of the findings when holding total protein intake constant, we fitted an additional model based on Model 6 (without energy intake), but with the inclusion of intakes of total protein (continuous), total carbohydrates (continuous), saturated fats (continuous) and unsaturated fats (continuous). Likelihood-ratio χ2 statistics were estimated at each adjustment level (for models 1–6) by comparing regression models with and without inclusion of the amino acids of interest using a likelihood ratio test [21]. The proportional hazards assumption was assessed using Schoenfeld residuals. The assumption was not met for two covariates in the models for ischaemic stroke (history of diabetes and prior hypertension) and for haemorrhagic stroke (prior hypertension and BMI). However, fitting these covariates as stratifying variables fulfilled the assumption and yielded close to identical estimates as adjusting, so they were retained as adjustment variables.
To investigate whether the observed associations for the amino acids were independent of the other amino acids, we included additional models mutually adjusting for each of the other dietary amino acids, one at a time. We also included post-hoc analyses adjusting the amino acids that remain significant across different models for its major food sources. To assess whether the associations were independent of blood pressure, we estimated the adjusted mean systolic and diastolic blood pressure by fifths of each dietary amino acid in the subset of participants with blood pressure measurements (n = 267,642), using the same covariate adjustments as above. Additionally, we repeated the Cox regression analyses in this subset (based on Model 5), with and without further adjustment for systolic or diastolic blood pressure.
As secondary analyses, we repeated the main analyses for the two subtypes of haemorrhagic stroke (intracerebral haemorrhage and subarachnoid haemorrhage). To assess potential heterogeneity of the associations by subgroups of the major confounders and to assess residual confounding, we also examined the results stratified by age at recruitment (< 55, 55–64, ≥ 65 years), sex, BMI (< 25, 25–29.9, ≥ 30 kg/m2), smoking status (never, former, current smokers), alcohol drinking (non-drinkers, moderate drinkers < 15 g/day, heavy drinkers ≥ 15 g/day) and history of diseases (as a dichotomy of no disease history vs history of diabetes, hypertension or hyperlipidaemia). We also examined the associations based on observed intakes stratified by EPIC country and pooled using a meta-analysis approach. Tests of heterogeneity of trend between subgroups were conducted by comparing the risk coefficients in each subgroup using inverse variance weighting, testing for statistical significance with a χ2 test on k − 1 degrees of freedom, where k is the number of subgroups. To assess whether the overall results might be influenced by reverse causation, we repeated the analyses for ischaemic and haemorrhagic stroke after excluding the first 4 years of follow-up. A complete case analysis was also performed to assess the potential influence of missing covariates, which were coded as a missing category in the main analyses.
All tests for statistical significance were two-sided. Conventional p values are reported, but results were interpreted with consideration of multiple testing. To account for multiple testing while allowing for correlation between the exposures, we conducted a principal component analysis of the exposure variables, and determined that the first four principal components explained 99% of the total variation in the exposure data [22, 23]. Consequently, the effective number of independent tests was determined to be four, and the statistical significance level after Bonferroni correction for multiple testing based on this number was defined as 0.05/4 = 0.0125. All analyses were performed using Stata version 17.0 (StataCorp, TX, USA). All figures were generated using “Jasper makes plots” package [24] version 2-266 in R version 4.2.1.
Results
Baseline and dietary characteristics
The baseline characteristics of the study participants are shown in Table 1 and additionally in detail in Supplementary table 1. Intakes of dietary amino acids, total protein and total energy are shown by sex in Supplementary table 2, and by EPIC country in Supplementary table 3. The Spearman correlation coefficients between the individual dietary amino acids are shown in Supplementary Fig. 2, and for amino acids with dietary protein and food sources of protein in Supplementary Figs. 3 and 4 respectively.
Associations of dietary amino acids with stroke risk
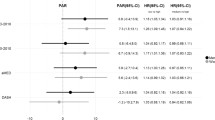
After a median follow-up of 12.9 years, there were 4295 cases of ischaemic stroke and 1375 cases of haemorrhagic stroke among 356,142 participants (4,529,626 person-years in total). In the fully adjusted models, associations between dietary amino acids and risks of ischaemic and haemorrhagic stroke were numerically similar irrespective of whether the amino acids were expressed as a percentage of total protein (Fig. 1, Supplementary tables 4–5), in g/day (Supplementary Fig. 5, Supplementary tables 4–5), g/1000 kcal (Supplementary Fig. 6, Supplementary tables 4–5), or as a percentage of total amino acids (Supplementary Fig. 7, Supplementary tables 4–5). Unless otherwise stated, the primary results reported on the associations that remained statistically significant after correcting for multiple testing, or results from categorical analyses where we observed non-linear associations.
Hazard ratios (95% confidence intervals) for ischaemic (4295 cases) and haemorrhagic stroke (1375 cases) by increments of calibrated intakes of dietary amino acids (as a percent of total protein). Hazard ratios modelled per 1 sex-specific SD increment in dietary amino acids, expressed as a percent of total protein. The model was stratified by sex and centre, and adjusted for age, calibrated energy intake, smoking, calibrated alcohol consumption, physical activity, employment status, highest level of education completed, history of diabetes, prior hypertension, prior hyperlipidaemia, body mass index. Estimates with an aserisk denote ones that were statistically significant after correcting for multiple testing
When expressing the amino acids as a percentage of total protein, in the fully adjusted models (Model 5) we found inverse associations of ischaemic stroke for each 1 SD higher calibrated intake of total and individual branched-chain amino acids (HR [95% CI] per SD higher of total: 0.93 [0.88, 0.98], isoleucine: 0.93 [0.88, 0.98], leucine: 0.93 [0.87, 0.98], valine: 0.92 [0.88, 0.97]), phenylalanine (0.92 [0.87, 0.98]), threonine (0.93 [0.88, 0.99]), tryptophan (0.94 [0.89, 0.99]), total non-essential amino acids (0.92 [0.87, 0.98]), glutamic acid (0.90 [0.84, 0.96]), proline (0.88 [0.82, 0.94]), serine (0.92 [0.88, 0.97]) and tyrosine (0.92 [0.88, 0.98]) (Fig. 1). Glycine showed a non-linear association with ischaemic stroke in the categorical analysis (HR [95% CI] 1.28 [1.14, 1.43] for top versus bottom fifth, p non-linearity < 0.001), but only when expressing glycine as a percentage of estimated intakes of total protein or total amino acids (Supplementary table 4). For haemorrhagic stroke, no statistically significant associations were observed in the continuous analyses after correcting for multiple testing (Fig. 1). In categorical analyses, lower risks were observed from the second to top fifth of proline intake compared to bottom fifth (top versus bottom fifth: 0.72 [0.58, 0.90], p-trend across categories = 0.006, Supplementary table 5). No statistically significant associations were observed for total protein with either ischaemic or haemorrhagic stroke (Supplementary Figs. 4–5, Supplementary tables 4–5), or in any analyses examining the two haemorrhagic stroke subtypes as outcomes (Supplementary table 6). Results from the different covariate adjustment models are shown in Supplementary tables 7–9.
Mutual adjustment of dietary amino acids
Results with mutual adjustment for each of the other amino acids for the ten amino acids that were inversely associated with ischaemic stroke risk, as reported above, are shown in Fig. 2 (for the five amino acids that were significantly associated in both the calibrated and observed analyses) and in Supplementary Fig. 8 (for the five amino acids that were significantly associated in the calibrated analyses only). The inverse associations for proline persisted after adjustment for all other amino acids, but the associations for other amino acids were attenuated upon adjustment for proline. However, the higher risk of ischaemic stroke comparing top to bottom fifth of glycine intake remained with adjustment for proline (1.27 [1.12, 1.43], results not shown in tables). In post-hoc analyses, the inverse associations for proline with ischaemic stroke remained upon mutual adjustment for its different food sources (e.g. 0.89; 0.83, 0.96 after mutual adjustment for cheese, with no significant association for cheese in the same model, results not shown in tables). Based on these findings, the changes in the HRs and likelihood ratio χ2 statistics for proline are also depicted graphically in Fig. 3, by each level of covariate adjustment.
Hazard ratios (95% confidence intervals) for the association between selected dietary amino acids and ischaemic stroke (4295 cases), with mutual adjustment for each of the other amino acids. Results are shown for amino acids which were statistically significantly associated with ischemic stroke in both calibrated and uncalibrated analyses. Hazard ratios modelled per 1 sex-specific SD (as shown in Fig. 1) increment in dietary amino acids in the column heading, expressed as a percent of total protein, with multivariable adjustment plus amino acid on the left panel. If the amino acid in the column and in the left panel are the same, the result is interpretable as the hazard ratio for the amino acid based on the multivariable-adjusted model
Hazard ratios (95% confidence intervals) for the association between dietary proline and ischaemic stroke (4295 cases), with additional adjustment for each covariate. Hazard ratios modelled per 1 SD increment (1 SD = 1.0 in men, 0.9 in women) in calibrated proline intake, expressed as a percent of total protein. The minimally adjusted model was stratified by sex and centre, and adjusted for age (continuous). The likelihood-ratio test statistic for proline is the χ2 statistic based on a likelihood ratio test comparing models with and without proline, at the varying levels of covariate adjustment. The changes in the χ2 statistic across models could be interpreted as a measure of the extent to which the covariates could account for any associations between proline and ischaemic stroke risk
Assessment of the role of blood pressure
The adjusted mean levels of systolic and diastolic blood pressure across fifths of dietary amino acids are shown in Supplementary tables 10–11. Across all amino acids, higher intakes were associated with higher blood pressure, though the differences were very small (up to 1 mmHg difference in most cases). As expected from these small differences, the results for both ischaemic and haemorrhagic stroke were almost unchanged after further adjustment for either systolic or diastolic blood pressure (Supplementary table 12).
Sensitivity analyses
In stratified analyses, the main findings were consistent between subgroups of age, sex, BMI, smoking status, alcohol drinking and disease status for both ischaemic and haemorrhagic stroke (Supplementary tables 13–24). The pooled estimates by country using a meta-analysis approach were similar (Supplementary tables 25–26), and results were consistent after excluding the first 4 years of follow-up (Supplementary table 27) or based on a complete case analysis (Supplementary table 28).
Discussion
In this large prospective study, we observed inverse associations of dietary intakes of proline with the risk of ischaemic stroke after adjustment of potential confounders, independent of the other amino acids and of total protein intake. The associations were consistent regardless of how the amino acids were modelled (i.e. percentage of protein, g/day and g/1000 kcal), and across all subgroup analyses. Although inverse associations with ischaemic stroke were initially observed for some other amino acids, these might be explained by the high correlations between these amino acids and proline, since the associations were attenuated after adjustment for proline. We further observed evidence of a higher risk of ischaemic stroke in individuals with the highest intakes of dietary glycine, and a lower risk of haemorrhagic stroke in people with higher intakes of dietary proline, though these associations differed depending on the way dietary amino acids were modelled in the analyses.
The present analyses provide important novel evidence on the associations between dietary amino acids and stroke risk, as only two prospective studies have previously published on the topic [5, 6]. The Swedish Mammography Cohort, with 1751 cases of total stroke (1311 ischaemic and 264 haemorrhagic after 10 years), reported an inverse association between dietary cysteine and the risk of total stroke, but no other associations including for proline were observed after mutual adjustment of the amino acids [5]. In contrast to that study, we did not find an association of cystine with either stroke subtype; however, although cystine is expected to be highly correlated with cysteine, their molecular structures are different, so the two sets of results are not directly comparable. The Takayama Study in Japan, with 677 deaths from stroke (393 ischaemic and 153 intracerebral haemorrhage after 16 years), showed that glutamic acid intake was inversely associated with total stroke mortality among women, while glycine intake was positively associated with ischaemic stroke mortality in men with no prior history of hypertension; the study did not report on proline [6]. In our study, we also observed an inverse association of glutamic acid intake with ischaemic stroke, but this association was attenuated when we further adjusted for dietary proline. In accordance with the Takayama Study, we also found a higher risk of ischaemic stroke for people with the highest fifth of glycine intake. The differences in intakes of amino acids across populations should be considered when comparing the findings. For example, the average intakes of both glutamic acid and glycine when expressed as a percentage of total protein in the EPIC study is closer to the lower end of the intakes reported in the Takayama study. Overall, compared to the two previous studies, the current study is over three- and ten-fold larger in case numbers. We also focused on examining ischaemic and haemorrhagic stroke separately, based on prior evidence of heterogeneity in risk factors by stroke subtypes. Overall, the associations of dietary amino acids with haemorrhagic stroke were less clear. As the number of haemorrhagic stroke cases in the study was comparatively low, further investigations in other populations with higher numbers of haemorrhagic strokes are warranted.
Previous studies have largely attributed the observed associations of dietary amino acids with stroke risk to the influences of the amino acids on blood pressure. The INTERMAP study reported inverse associations of glutamic acid and to a lesser degree proline [18], but positive associations of glycine intake [25], with blood pressure. Our results did not suggest an important role of blood pressure in explaining any of the findings, given that we did not observe any meaningful differences in blood pressure by amino acid intake, nor did we observe any differences in the HRs after further adjusting for blood pressure. However, blood pressure and diet in EPIC were both measured at recruitment, which does not allow the evaluation of a temporal association. Additionally, the use of blood pressure measurements assessed at a single time point as an indicator of long-term blood pressure is also prone to random measurement error, which could result in an underestimation of its role in explaining any associations between amino acid intakes and stroke risk. Furthermore, blood pressure measurements in EPIC were not taken using a standardised protocol across centres [26], but our analyses were stratified by centre, so any influence on the results should be small.
The possible biological mechanisms for the association between proline and stroke risk are not yet well defined. Despite being non-essential amino acids, proline, glycine and hydroxyproline are the major constituents of collagen, which accounts for about one-third of proteins in the human body [27]. Collagen has an important role in maintaining the structure and strength of connective tissues, cartilage and blood vessels [27, 28], and studies from animal models have suggested that endogenous synthesis of these amino acids may not be adequate for growth or collagen production [27, 29]. Because proline is the only precursor for hydroxyproline, which in turn is a precursor for glycine [27, 30], dietary intakes of proline may be of particular importance for collagen synthesis. However, while collagen likely has an integral role in the pathogenesis of haemorrhagic stroke due to its importance in the maintenance of vascular integrity, its relevance for ischaemic stroke, which is typically caused by a blood clot in the brain, is less clear, but nonetheless has been suggested [31]. Overall, the relevance of collagen and other amino acids that are also precursors for collagen synthesis in the pathogenesis of ischaemic stroke requires further investigation.
In contrast to other amino acids, proline contains an imino group in its structure instead of a primary amino group, which results in its distinctive cyclic structure and exclusion from standard amino acid metabolism [32, 33]. For this reason, the family of proline-containing peptides, collectively called glycoprolines, cannot be degraded by most peptidases owing to the presence of proline in their structures, and thus are more likely to enter the bloodstream and exert various biological effects, including possible atheroprotective or neuroprotective roles, regulation of insulin-like growth factor-I (IGF-I) homeostasis and lipid metabolism including lowering of total cholesterol [34]. Glycoprolines may be derived from collagen or IGF-I; a study in rats has shown that oral administration of cyclic glycine-proline, a glycoproline derived from IGF-I, promoted neural plasticity and remodelling in animals with focal ischaemic lesions, suggesting a neuroprotective effect of cyclic glycine-proline against ischaemic stroke or its recovery [35]. In human studies, both cyclic glycine-proline concentrations [36] and proline concentrations [37,38,39] have been proposed as prognostic markers of stroke recovery or stroke events. However, the directions of the changes observed have been inconsistent. Other studies have also reported low correlations between dietary intake and circulating levels of proline [40]. Therefore, further research into the link between proline intake and the physiological effects of circulating proline, as well as the role of proline in both stroke incidence and prognosis (recovery or mortality) is needed.
Among dietary sources, the results of the present study showed that dairy products, especially cheese, was most highly correlated with proline intake. Therefore, the findings of lower risks of ischaemic stroke in people with higher proline intake in the present study are consistent with previous observations of lower ischaemic stroke risk in people with higher intake of cheese and yogurt in the same cohort [7]. Despite being a major source of saturated fat, higher intakes of dairy products have been inversely associated with cardiovascular disease outcomes in a meta-analysis of prospective studies [41], suggesting that some components in cheese or dairy products might be protective. For example, studies have suggested that the specific saturated fatty acid isomer composition of different foods [42], or the calcium [43] or probiotics content [44] of dairy foods may account for their associations with stroke risk. As the observed association for proline remained upon adjustment for cheese and other food sources, the findings of the present study suggest that the amino acid profiles of dairy products and their associations with CVD warrant further research. Meanwhile, dietary intake of glycine was strongly correlated with red meat intake, which was previously found to be modestly but positively associated with ischaemic stroke risk in EPIC, though this association was attenuated after adjustment for fibre intake [7]. Further population-based and mechanistic studies are needed to replicate the observed associations between dietary amino acids and risks of stroke subtypes, prior to investigating any implications for potential dietary interventions.
The chief strengths of this study include the large sample size with participants recruited from seven European countries, the prospective design and prolonged duration of follow-up. EPIC is also a well-characterised cohort with extensive dietary and lifestyle/health behavioural data, allowing us to examine estimated intakes of 19 dietary amino acids with stroke risk while adjusting for multiple confounders. The ascertainment of stroke cases in this cohort was either completely or partially validated depending on the EPIC country, and previous dietary analyses have found no heterogeneity in the associations by the extent of stroke validation [7]. To adjust for potential bias due to differences between the country-specific dietary questionnaires, we also applied calibration to the dietary data using additional standardised 24-h recalls to reduce between-centre heterogeneity and to reduce measurement error. The study also had several limitations. Diet was ascertained using a single dietary questionnaire collected at baseline, which meant that we were not able to account for dietary changes during follow-up. We were unable to assess the associations of dietary glutamine, asparagine or cysteine with stroke risk, as estimates of these amino acids were not available from the USDA database, while glutamic acid and aspartic acid may be overestimated [15, 16]. The matching of amino acids data based on a single US database in different countries across Europe may also be a limitation, and the absolute validity of the amino acid estimates assessed using FFQs and 24-h recalls is not known, although good agreement and thus relative validity has been shown for estimates of total energy and protein intakes between the USDA and country-specific databases in the previous validation study [14]. Because of the large number of tests conducted, false positive findings due to chance are possible, but we focused on the findings that survived correction for multiple testing. We also conducted analyses further adjusting for each one of the other amino acids, which given their high correlations might constitute over-adjustment, but this approach also helped to highlight the clear associations with proline. As with any observational study, the present study could not establish causality, and cannot fully exclude residual confounding or reverse causation. However, we assessed the former by comparing the changes in χ2 statistics across models and limited the latter possibility by our sensitivity analysis excluding the first four years of follow-up. Information on other potential mediators such as cholesterol concentrations was only available in a small subset of participants at the study baseline, so we were unable to access their potential effects. Finally, the present study was largely restricted to white European individuals, therefore the findings may not be generalisable to other populations which have different sources of protein intake and dietary amino acid profiles, as well as genetic differences.
Conclusions
This large study demonstrated an inverse association of both absolute intake and relative proportions of dietary proline with the risk of ischaemic stroke, independent of the other amino acids. The suggestive associations of lower risk of haemorrhagic stroke in people with higher proline intake, and higher risk of ischaemic stroke in people with higher proportions of glycine intake, also warrant further investigation. These observations were largely unexplained by differences in blood pressure. Further research is needed from both large-scale population-based studies and mechanistic studies to replicate these findings, in addition to genetic studies and intervention trials to assess the causal relevance of these associations and possible implications for prevention.
Data availability
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.
References
Feigin VL, Stark BA, Johnson CO et al (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20:1–26. https://doi.org/10.1016/S1474-4422(21)00252-0
Zhang Z, Xu G, Yang F et al (2014) Quantitative analysis of dietary protein intake and stroke risk. Neurology 83:19–25. https://doi.org/10.1212/WNL.0000000000000551
Zhang XW, Yang Z, Li M et al (2016) Association between dietary protein intake and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol 223:548–551. https://doi.org/10.1016/j.ijcard.2016.08.106
Górska-Warsewicz H, Laskowski W, Kulykovets O et al (2018) Food products as sources of protein and amino acids-the case of Poland. Nutrients. https://doi.org/10.3390/nu10121977
Larsson SC, Håkansson N, Wolk A (2015) Dietary cysteine and other amino acids and stroke incidence in women. Stroke 46:922–926. https://doi.org/10.1161/STROKEAHA.114.008022
Nagata C, Wada K, Tamura T et al (2015) Dietary intakes of glutamic acid and glycine are associated with stroke mortality in Japanese adults. J Nutr 145:720–728. https://doi.org/10.3945/jn.114.201293
Tong TYN, Appleby PN, Key TJ et al (2020) The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur Heart J 44:1–11. https://doi.org/10.1093/eurheartj/ehaa007
Sun L, Clarke R, Bennett D et al (2019) Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med 25:569–574. https://doi.org/10.1038/s41591-019-0366-x
Price AJ, Wright FL, Green J et al (2018) Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology 90:e298–e306. https://doi.org/10.1212/WNL.0000000000004856
Riboli E, Hunt K, Slimani N et al (2002) European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 5:1113. https://doi.org/10.1079/PHN2002394
Danesh J, Saracci R, Berglund G et al (2007) EPIC-Heart: The cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol 22:129–141. https://doi.org/10.1007/s10654-006-9096-8
Slimani N, Ferrari P, Ocké M et al (2000) Standardization of the 24-hour diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr 54:900–917
Ferrari P, Day NE, Boshuizen HC et al (2008) The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol 37:368–378. https://doi.org/10.1093/ije/dym242
Iguacel I, Perez-Cornago A, Schmidt JA et al (2022) Evaluation of protein and amino acid intake estimates from the EPIC dietary questionnaires and 24-h dietary recalls using different food composition databases. Nutr Metab Cardiovasc Dis 32:80–89. https://doi.org/10.1016/j.numecd.2021.09.012
Lenders CM, Liu S, Wilmore DW et al (2009) Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur J Clin Nutr 63:1433–1439. https://doi.org/10.1038/ejcn.2009.110
Mustățea G, Ungureanu EL, Iorga E (2019) Protein acidic hydrolysis for amino acids analysis in food—progress over time: a short review. J Hyg Eng Des 26:81–87
National Center for Biotechnology Information (2022) PubChem Compound Summary for CID 595, Cystine. https://pubchem.ncbi.nlm.nih.gov/compound/Cystine. Accessed 11 Feb 2022
Stamler J, Brown IJ, Daviglus ML et al (2009) Glutamic acid, the main dietary amino acid, and blood pressure: The intermap study (international collaborative study of macronutrients, micronutrients and blood pressure). Circulation 120:221–228. https://doi.org/10.1161/CIRCULATIONAHA.108.839241
Tong TYN, Bradbury KE, Key TJ (2019) RE: “Associations of dietary protein intake with fat-free mass and grip strength: a cross-sectional study in 146,816 uk biobank participants.” Am J Epidemiol 188:10. https://doi.org/10.1093/aje/kwz029
Ibsen DB, Laursen ASD, Würtz AML et al (2021) Food substitution models for nutritional epidemiology. Am J Clin Nutr 113:294–303. https://doi.org/10.1093/ajcn/nqaa315
Floud S, Balkwill A, Moser K et al (2016) The role of health-related behavioural factors in accounting for inequalities in coronary heart disease risk by education and area deprivation: prospective study of 1.2 million UK women. BMC Med 14:145. https://doi.org/10.1186/s12916-016-0687-2
Gao X, Starmer J, Martin ER (2008) A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 32:361–369. https://doi.org/10.1002/gepi.20310
Adams CD, Richmond R, Santos Ferreira DL et al (2019) Circulating metabolic biomarkers of screen-detected prostate cancer in the ProtecT study. Cancer Epidemiol Biomarkers Prev 28:208–216. https://doi.org/10.1158/1055-9965.EPI-18-0079
Arnold M (2022) Jasper: Jasper makes plots. 2020
Stamler J, Brown IJ, Daviglus ML et al (2013) Dietary glycine and blood pressure: the international study on macro/micronutrients and blood pressure. Am J Clin Nutr 98:136–145. https://doi.org/10.3945/ajcn.112.043000
Schulze MB, Kroke A, Saracci R, Boeing H (2002) The effect of differences in measurement procedure on the comparability of blood pressure estimates in multi-centre studies. Blood Press Monit 7:95–104. https://doi.org/10.1097/00126097-200204000-00002
Li P, Wu G (2018) Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38. https://doi.org/10.1007/s00726-017-2490-6
Xu J, Shi G-P (2014) Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842:2106–2119. https://doi.org/10.1016/j.bbadis.2014.07.008
Hou Y, Yao K, Yin Y, Wu G (2016) Endogenous synthesis of amino acids limits growth, lactation, and reproduction in animals. Adv Nutr 7:331–342. https://doi.org/10.3945/an.115.010850
Wu G, Bazer FW, Burghardt RC et al (2011) Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 40:1053–1063. https://doi.org/10.1007/s00726-010-0715-z
Yao Y (2019) Basement membrane and stroke. J Cereb Blood Flow Metab 39:3–19. https://doi.org/10.1177/0271678X18801467
Phang JM, Donald SP, Pandhare J, Liu Y (2008) The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35:681–690. https://doi.org/10.1007/s00726-008-0063-4
Wyse ATS, Netto CA (2011) Behavioral and neurochemical effects of proline. Metab Brain Dis 26:159–172. https://doi.org/10.1007/s11011-011-9246-x
Misiura M, Miltyk W (2019) Proline-containing peptides-new insight and implications: a review. BioFactors 45:857–866. https://doi.org/10.1002/biof.1554
Kaneko H, Namihira M, Yamamoto S et al (2022) Oral administration of cyclic glycyl-proline facilitates task learning in a rat stroke model. Behav Brain Res 417:113561. https://doi.org/10.1016/j.bbr.2021.113561
Fan D, Krishnamurthi R, Harris P et al (2019) Plasma cyclic glycine proline/IGF-1 ratio predicts clinical outcome and recovery in stroke patients. Ann Clin Transl Neurol 6:669–677. https://doi.org/10.1002/acn3.743
Goulart VAM, Sena MM, Mendes TO et al (2019) Amino acid biosignature in plasma among ischemic stroke subtypes. Biomed Res Int. https://doi.org/10.1155/2019/8480468
Szpetnar M, Hordyjewska A, Malinowska I et al (2016) The fluctuation of free amino acids in serum during acute ischemic stroke. Curr Issues Pharm Med Sci 29:151–154. https://doi.org/10.1515/cipms-2016-0031
Wang X, Zhang L, Sun W et al (2021) Changes of metabolites in acute ischemic stroke and its subtypes. Front Neurosci 14:1–8. https://doi.org/10.3389/fnins.2020.580929
Iguacel I, Schmidt JA, Perez-Cornago A et al (2021) Associations between dietary amino acid intakes and blood concentration levels. Clin Nutr 40:3772–3779. https://doi.org/10.1016/j.clnu.2021.04.036
Alexander DD, Bylsma LC, Vargas AJ et al (2016) Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr 115:737–750. https://doi.org/10.1017/S0007114515005000
Steur M, Johnson L, Sharp SJ et al (2021) Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: findings from the EPIC-CVD case-cohort study across nine European countries. J Am Heart Assoc. https://doi.org/10.1161/JAHA.120.019814
Lorenzen JK, Astrup A (2011) Dairy calcium intake modifies responsiveness of fat metabolism and blood lipids to a high-fat diet. Br J Nutr 105:1823–1831. https://doi.org/10.1017/S0007114510005581
Mozaffarian D, Wu JHY (2018) Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res 122:369–384. https://doi.org/10.1161/CIRCRESAHA.117.309008
Acknowledgements
The authors thank all participants in the EPIC cohort for their invaluable contribution to the study.
Funding
This work was supported by a Nuffield Department of Population Health Intermediate Fellowship (TYNT), the UK Medical Research Council (MR/M012190/1), Cancer Research UK (C8221/A19170 and 570/A16491), and the Wellcome Trust (Our Planet Our Health, Livestock Environment and People 205212/Z/16/Z). EPIC-CVD has been supported by the European Union Framework 7 (HEALTH-F2-2012-279233), the European Research Council (268834), the UK Medical Research Council (G0800270 and MR/L003120/1), the British Heart Foundation (SP/09/002 and RG/08/014 and RG13/13/30194), and the UK National Institute of Health Research. The establishment of the study subcohort was supported by the EU Sixth Framework Programme (FP6) (grant LSHM_CT_2006_037197 to the InterAct project) and the Medical Research Council Epidemiology Unit (grants MC_UU_12015/1 and MC_UU_12015/5). The coordination of the European Prospective Investigation into Cancer and Nutrition (EPIC) is financially supported by the International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF) (The Netherlands), and Statistics Netherlands is acknowledged for providing the causes of death; Health Research Fund (FIS)—Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology—ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten and the funds supporting the Northern Sweden Diet Database (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford) (United Kingdom). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. For the purpose of open access, the author(s) has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by TYNT and TJK with input from all other authors. TYNT analysed the data and wrote the first draft of the manuscript, with input from TJK, RC, JAS, IH, UN, NGF, FI, RCT, EW, KA, CCD and YTVS. All other authors provided the data and revised the manuscript critically for important intellectual content. TYNT had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tong, T.Y.N., Clarke, R., Schmidt, J.A. et al. Dietary amino acids and risk of stroke subtypes: a prospective analysis of 356,000 participants in seven European countries. Eur J Nutr 63, 209–220 (2024). https://doi.org/10.1007/s00394-023-03251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03251-4