Abstract
Purpose
We recently reported that fermentable non-digestible carbohydrates including fructo-oligosaccharides (FOS) commonly elevate colonic alkaline phosphatase (ALP) activity and the expression of IAP-I, an ALP gene, in rats fed a high-fat (HF) diet, and also elevate gut mucins and modulate gut microbiota. This study aims to investigate whether dietary fat types influence the effect of FOS on colonic ALP activity and the luminal environment in HF-fed rats.
Methods
Male Sprague–Dawley rats were fed a diet containing 30% soybean oil, corn oil, olive oil or lard with or without 4% FOS for 2 weeks. Colon ALP activity, gene expression, and gut luminal variables including mucins and microbiota were measured.
Results
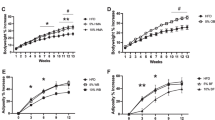
In the lard diet groups, dietary FOS significantly elevated colonic ALP activity and the expression of IAP-I. The elevating effect of FOS on colonic ALP activity was also observed in the olive oil diet groups, although here the IAP-I expression was not changed. However, the soybean oil and corn oil diet groups did not exhibit the elevating effect of FOS on colon ALP. Fecal ALP and mucins were significantly elevated by dietary FOS regardless of dietary fat types, and the effect of FOS was prominent in the lard diet groups. The number of Lactobacillus spp. observed in fecal matter was significantly increased by dietary FOS in the lard and olive oil diet groups, but not in the soybean oil and corn oil diets groups.
Conclusion
This study suggests that dietary fat types may change the effect of FOS on the colonic luminal environment including the ALP activity in rats fed a high-fat diet.



Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- FOS:
-
Fructo-oligosaccharides
- HF:
-
High-fat
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids
References
Kudoh K, Shimizu J, Ishiyama A et al (1999) Secretion and excretion of immunoglobulin A to cecum and feces differ with type of indigestible saccharides. J Nutr Sci Vitaminol 45:173–181
Ito H, Satsukawa M, Arai E et al (2009) Soluble fiber viscosity affects both goblet cell number and small intestine mucin secretion in rats. J Nutr 139:1640–1647
Watzl B, Girrbach S, Roller M (2005) Inulin, oligofructose and immunomodulation. Br J Nutr 93:S49–S55
Ehara T, Izumi H, Tsuda M et al (2016) Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice. Br J Nutr 116:270–278
Goldberg RF, Austen WG Jr, Zhang X et al (2008) Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA 105:3551–3556
Lallès JP (2019) Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr Rev 77:710–724
Okazaki Y, Katayama T (2017) Glucomannan consumption elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, which is associated with increased production of protective factors for gut epithelial homeostasis in high-fat diet-fed rats. Nutr Res 43:43–50
Okazaki Y, Katayama T (2019) Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br J Nutr 121:146–154
Deplancke B, Gaskins HR (1141S) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73:1131S–1141S
Nyman M (2002) Fermentation and bulking capacity of indigestible carbohydrates: the case of inulin and oligofructose. Br J Nutr 87:S163–S168
DeCoffe D, Quin C, Gill SK et al (2016) Dietary lipid type, rather than total number of calories, alters outcomes of enteric infection in mice. J Infect Dis 213:1846–1856
Ghosh S, Molcan E, DeCoffe D, Dai C, Gibson DL (2013) Diets rich in n–6 PUFA induce intestinal microbial dysbiosis in aged mice. Br J Nutr 110:515–523
Ghosh S, DeCoffe D, Brown K, Rajendiran E, Estaki M, Dai C, Yip A, Gibson DL (2013) Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs lps dephosphorylation activity causing sepsis. PLoS ONE 8:e55468
Kishino S, Takeuchi M, Park SB et al (2013) Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci USA 110:17808–17813
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Lima GC, Vieira VCC, Cazarin CBB (2018) Fructooligosaccharide intake promotes epigenetic changes in the intestinal mucosa in growing and ageing rats. Eur J Nutr 57:1499–1510
Abell LL, Levy BB, Brodie BB, Kendall FE (1952) A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem 195:357–366
Pearson S, Stern S, McGavack TH (1953) Rapid, accurate method for determination of total chlolesterol in serum. Anal Chem 25:813–814
Sogabe N, Maruyama R, Goseki-Sone M (2007) Influence of long-term lactose feeding on intestinal alkaline phosphatase in rats. J Home Econ Jpn 58:255–260
Narisawa S, Hoylaerts MF, Doctor KS et al (2007) A novel phosphatase upregulated in Akp3 knockout mice. Am J Physiol Gastrointest Liver Physiol 293:G1068–G1077
Harada T, Koyama I, Kasahara T (2003) Heat shock induces intestinal-type alkaline phosphatase in rat IEC-18 cells. Am J Physiol Gastrointest Liver Physiol 284:G255–G262
Dharmani Poonam Strauss J, Ambrose C, Allen-Vercoe E et al (2011) Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun 79:2597–2607
Bovee-Oudenhoven IM, Termont DS, Heidt PJ et al (1997) Increasing the intestinal resistance of rats to the invasive and calcium. Gut 40:497–504
Morita T, Tanabe H, Sugiyama K et al (2004) Dietary resistant starch alters the characteristics of colonic mucosa and exerts a protective effect on trinitrobenzene sulfonic acid-induced colitis in rats. Biosci Biotechnol Biochem 68:2155–2164
Growther RS, Wetmore RF (1987) Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem 163:170–174
Nadatani Y, Watanabe T, Suda W et al (2019) Gastric acid inhibitor aggravates indomethacin-induced small intestinal injury via reducing Lactobacillus johnsonii. Sci Rep 9:17490
Yang Y, Sitanggang NV, Kato N et al (2015) Beneficial effects of protease preparations derived from Aspergillus on the colonic luminal environment in rats consuming a high-fat diet. Biomed Rep 3:715–720
Fernandes J, Rao AV, Wolever TM (2000) Different substrates and methane producing status affect short-chain fatty acid profile produced by in vitro fermentation of human feces. J Nutr 130:1932–1936
Okazaki Y, Tomotake H, Tsujimoto K et al (2011) Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucins, and cecal organic acids in rats fed a high-fat diet. J Nutr 141:1975–1981
Chen HM, Lifschitz CH (1989) Preparation of fecal samples for assay of volatile fatty acids by gas-liquid chromatography and high-performance liquid chromatography. Clin Chem 35:74–76
Abulizi N, Quin C, Brown K, Chan YK, Gill SK, Gibson DL (2019) Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 11:E418
Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, Xaus J, Zarzuelo A (2005) Dietary olive oil supplemented with fish oil, rich in epa and dha (n–3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with dss-induced colitis. J Nutr 135:687–694
Tjonneland A, Overvad K, Bergmann MM et al (2009) Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut 58:1606–1611
Polan CE, McNeill JJ, Tove SB (1964) Biohydrogenation of unsaturated fatty acids by rumen bacteria. J Bacteriol 88:1056–1064
Gomes JR, Ayub LC, Dos Reis CA et al (2017) Goblet cells and intestinal Alkaline phosphatase expression (IAP) during the development of the rat small intestine. Acta Histochem 119:71–77
Zarepour M, Bhullar K, Montero M et al (2013) The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 81:3672–3683
Chu YH, Yu XX, Jin X, Wang YT, Zhao DJ, Zhang P, Sun GM, Zhang YH (2019) Purification and characterization of alkaline phosphatase from lactic acid bacteria. RSC Adv 9:354–360
Malo MS, Moaven O, Muhammad N et al (2014) Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol 306:G826–G838
Hino S, Takemura N, Sonoyama K et al (2012) Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats. J Nutr 142:1429–1436
Windey K, Preter VD, Verbeke K (2012) Relevance of protein fermentation to gut health. Mol Nutr Food Res 56:184–196
Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ (1979) The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32:2094–2101
Juśkiewicz J, Zduńczyk Z, Wróblewska M (2005) The effect of the administration of cellulose and fructans with different degree of polymerization to rats on caecal fermentation and biochemical indicators in the serum. Czech J Anim Sci 50:273–280
Le Leu RK, Brown IL, Hu Y, Morita T, Esterman A, Young GP (2007) Effect of dietary resistant starch and protein on colonic fermentation and intestinal tumourigenesis in rats. Carcinogenesis 28:240–245
Acknowledgements
This research was supported by JSPS KAKENHI Grant Number 18K02244.
Author information
Authors and Affiliations
Contributions
YO and TK conceived, designed and performed the experiments. YO analyzed the data. YO and TK wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Experimental procedures were reviewed and approved by the ethics committee for Animal experimentation of the Fuji Women’s University (approval no. 2018-2). All animal experiments were conducted according to the Guidelines for Animal experiments of the Fuji Women’s University, “Japanese Act on Welfare and Management of Animals” and “Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain”. No adverse events were observed during this study.
Rights and permissions
About this article
Cite this article
Okazaki, Y., Katayama, T. The effects of different high-fat (lard, soybean oil, corn oil or olive oil) diets supplemented with fructo-oligosaccharides on colonic alkaline phosphatase activity in rats. Eur J Nutr 60, 89–99 (2021). https://doi.org/10.1007/s00394-020-02219-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02219-y