Abstract
Purpose
A diet rich in berries is believed to play a distinct role in the prevention of metabolic diseases associated with obesity. So far, there have been no published clinical observations evaluating the influence of Aronia melanocarpa on hemostasis. The aim of our study was to investigate the effects of A. melanocarpa extract (AM) supplementation on platelet aggregation, clot formation, and lysis in patients with metabolic syndrome (MS).
Methods
Middle-aged non-medicated subjects with MS (n = 38) and 14 healthy volunteers were included in this study. Patients with MS were treated with 100 mg of AM three times daily for 2 months.
Results
We observed a significant reduction in the concentration of TC, LDL-C, and TG after AM supplementation. Beneficial changes in coagulation parameters were also observed. After 1 month of AM administration, we noticed significant inhibition of platelet aggregation. However, this effect became less pronounced after 2 months of supplementation. In the case of coagulation induced by endogenic thrombin, a significant decrease in the overall potential for coagulation was induced after 1 or 2 months of supplementation. Moreover, after 1 month of AM extract supplementation, we observed a beneficial reduction in the overall potential for clot formation and fibrinolysis.
Conclusions
We observed the normalization of hemostasis parameters in MS patients after both 1 and 2 months of AM administration. After 1 month of AM supplementation, we found favorable changes in regards to the overall potential for plasma clotting, clot formation, and lysis, as well as in the lipid profiles of subjects.
Similar content being viewed by others
Introduction
A diet rich in berries is thought to play an important role in the prevention of cardiovascular disease and cancer. It is believed that a high dietary intake of berries, especially Aronia melanocarpa (which is rich in polyphenols, such as anthocyanins, procyanidins, and flavonoids), may inhibit atherosclerotic plaque progression. Cyanidin glycosides are believed to play a particularly important role in the prevention of metabolic diseases associated with obesity. There is evidence that cyanidin glycosides increase adiponectin secretion in white fat tissue via AMP-dependent kinase induction [1]. Anthocyanins also possess several beneficial features. Similar to other polyphenols, they show strong antioxidant effects, suppress inflammatory processes, and prevent damage to the vascular endothelium [2]. Both in vitro and in vivo studies show promising results in regards to the role of anthocyanins in preventing obesity and ameliorating hyperglycemia and adipocyte function [3]. Anthocyanins also positively influence the circulatory system by lowering blood pressure and by maintaining the proper permeability and elasticity of arterial vessels [4, 5].
In metabolic syndrome, also known as syndrome X or insulin resistance syndrome, the presence of dangerous risk factors for cardiovascular disease (including abdominal obesity, lipid disorders, and atherogenic dyslipidemia) is combined with impaired glucose tolerance and elevated blood pressure. Patients with MS have a threefold greater risk of acute coronary syndrome, a twofold greater risk of death, and a fivefold greater risk of developing type 2 diabetes. Enhanced serum levels of pro-inflammatory cytokines (IL-6 and TNF-α) and C-reactive protein (CRP) have been observed in patients with metabolic syndrome, along with microalbuminuria and an increase in coagulability [2, 6]. Several studies have shown that obese patients (when compared to non-obese controls) have higher plasma concentrations of all pro-thrombotic factors (fibrinogen, von Willebrand factor—vWF, and factor VII), as well as higher plasma levels of plasminogen activator inhibitor-1 (PAI-1) [7]. A positive association has been found to exist between central obesity and the changes mentioned above. It has been proposed that the secretion of IL-6 by adipose tissue, combined with the actions of adipose tissue expressed TNF-alfa in obese individuals, might be the basis for the association between insulin resistance, endothelial dysfunction, coagulopathy, and coronary heart disease. Finally, some hormonal abnormalities (pertaining to androgens and catecholamines) associated with the accumulation of body fat may contribute to the impairment of the coagulative pathway in obesity [6, 7].
The aim of our study was to investigate the effects of A. melanocarpa extract supplementation on platelet aggregation, clot formation, and lysis in patients with metabolic syndrome.
Subjects and methods
Reagents
We used adenosine 5′-diphosphate (ADP) produced by Sigma-Aldrich (Munich, Germany), thrombin produced by Biomed (Lublin, Poland), and recombinant tissue plasminogen activator (t-PA) produced by Boehringer (Ingelheim, Germany). Tris-buffered saline (TBS) and calcium chloride were obtained from Polish Chemical Reagents (Gliwice, Poland). A. melanocarpa extract (AM) was purchased from Agropharm SA (Poland).
Subjects
The study included 52 subjects (42–65 years old) subdivided into two groups. The study group (n = 38; 22 women and 16 men) included patients with metabolic syndrome. The control group consisted of 14 healthy volunteers (9 women and 5 men) matched for age and sex. Patients were eligible for the study if they met the following criteria: (1) waist circumference ≥80 cm for women and ≥94 cm for men, (2) TG level >150 mg/dL (1.7 mmol/L), (3) HDL-C level <40 mg/dL (1.0 mmol/L) for men and <50 mg/dL (1.3 mmol/L) for women. The exclusion criteria consisted of (1) secondary dyslipidemia in the course of autoimmune disorders, thyroid disease, chronic pancreatitis, nephritic syndrome, liver or biliary tract disease, or alcoholism, (2) any acute or chronic inflammatory processes, (3) congestive heart failure, (4) coronary artery disease, (5) history of myocardial infarction, stroke, or intermittent claudication, (6) moderate or severe arterial hypertension (WHO/ISH grade 2 or 3), (7) diabetes mellitus, (8) impaired renal or hepatic function, (9) malabsorption syndromes, (10) malignancy or history of malignancy, (11) treatment with hypolipemic, hypotensive, anticoagulant, antiplatelet, or profibrinolytic drugs, (12) concomitant treatment with drugs that may affect inflammatory processes in the vascular wall, including non-steroidal anti-inflammatory drugs, and angiotensin-converting enzyme inhibitors, (13) antioxidant therapy, (14) ongoing hormonal replacement therapy or oral contraception, (15) abuse of alcohol, (16) cigarette smoking, and (17) poor patient compliance.
Ethics
All subjects gave their written informed consent prior to participating in the study. The study was approved by the Bioethics Commission of the Medical University of Lodz (No. 241/06/KB).
Study design
Subjects with MS were treated with 100 mg of A. melanocarpa extract three times daily during the 2-month study period. This extract contained ca. 60 mg of total polyphenols, including a minimum of 20 mg of anthocyanins: 3-O-cyanidin-galactoside (64.5%), 3-O-cyanidin-arabinoside (28.9%), 3-O-cyanidin-xyloside (4.2%), and 3-O-cyanidinglucoside (2.4%). All participants of this study were on the low-fat diet (started 3 months before initiating active treatment with A. melanocarpa extract and continued till the end of the experiment). 30% of an individual’s daily calories originated from fat, including no more than 10% of calories from saturated fat. Participants were instructed not to modify their usual food intake and physical activity during the study. Additionally, ingestion of products containing black chokeberry (juices, jams, fresh, or frozen fruits) was prohibited. Three control visits were scheduled for the subjects: before initiation of treatment, after 1 month of therapy, and after 2 months of therapy. During the control visits, subjects underwent clinical examination, measurement of body weight and waist circumference, urine analysis, and venous blood sampling in order to evaluate the studied parameters and the safety of laboratory parameters. The following parameters were analyzed in blood samples: total and differential blood cell count, blood sedimentation rate, alanine and aspartate aminotransferases, electrolytes, bilirubin, creatinine, and total proteins. Blood samples were taken after an overnight fast in a quiet, temperature-controlled room (24–25 °C) between 8:00 and 9:00 a.m., in order to avoid circadian fluctuations. The samples were immediately coded so that the person performing the laboratory assay was unaware of the subject’s identity and study sequence. Compliance was assessed during each visit by tablet counts and was considered satisfactory when the number of tablets taken by the patient ranged from 90 to 100%.
Sample preparation
Blood was collected into Vacuette Coagulation Tubes (Greiner Bio-One, Austria) containing 3.2% buffered sodium citrate. Platelet-rich plasma (PRP) was obtained by blood centrifugation (at 150×g, for 10 min, at room temperature). Platelet-poor plasma (PPP) was obtained by subsequent centrifugation of the PRP (2,500×g, 20 min, 4 °C). PPP was stored at −70 °C until the measurements were taken. The platelet count in the PRP was adjusted to 1.8 × 108 platelets/mL. To preserve the discoid shape of platelets, the PRP was restored in a water bath at 37 °C during a resting period of 30 min.
Platelet aggregation assay
The aggregation of platelets in the PRP was measured with Born’s turbidimetric method [8] using a spectrophotometer (Cecil CE2021, London, England) at 37 °C and with stirring. Aggregation curves were induced by the addition of ADP (10 μmol/L) and were recorded and evaluated by our own computer program [9]. Our program estimated 5 parameters of platelet aggregation: maximal aggregation (A max), initial velocity (v 0), the time needed to reach maximal aggregation (T max), the aggregation level after 5 min (A 5min) from A max (which enabled us to estimate disaggregation), and platelet shape change (PSC, which is only a rough estimation of this process).
Test of clot formation and lysis (CL-test)
For this study, we used the CL-test (previously described by us), in conjunction with our own computer program [10]. This test is based on the evaluation of the global assay of coagulation and fibrinolysis by measuring changes in optical transmittance. To obtain a clotting and lysis curve, thrombin (final concentration 0.5 IU/mL) and t-PA (final concentration, 60 ng/mL) were added to three-time diluted PPP with TBS buffer. The addition of small amounts of thrombin (0.025 IU/mL) and calcium chloride (0.01 mmol/mL) does not result in coagulation initiation, but rather induces a feedback reaction that leads to endogenous thrombin generation, and as such, to clot formation. Curves were recorded continuously using a spectrophotometer (Cecil CE2021, London, England) at 37 °C and with stirring. They were then evaluated by the computer program. The following kinetic parameters of the examined processes were calculated: C AUC—overall potential of coagulation, TGt—thrombin generation time, CLAUC—overall potential of clot formation and lysis—phase I (clot formation): Tt—thrombin time, F max—maximum clotting, Tf—plasma clotting time, Fvo—initial plasma clotting velocity, Sf—area under the clot formation curve; phase II (forming a stable): Tc—clot stabilization time, Sc—area under the curve of stable clot formation; and phase III (fibrinolysis): L max—maximum lysis, Tl—fibrinolysis time, Lvo—initial clot fibrinolysis velocity, Sl—area under the fibrinolysis curve.
Other laboratory assays
The other laboratory parameters were determined with routinely used methods at the Dr. W. Bieganski Voivodship Specialistic Hospital in Lodz. Lipid profile: total serum cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) were determined by means of enzymatic methods (Olympus AU 400 analyzer; reagent kits produced by bioMerieux). Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedewald’s formula [11]. Fibrinogen levels were measured according to the Clauss method [12] with the use of reagents supplied by Fibriquik™ and the Coag-A-Mate MTX analyzer (bioMerieux).
Statistics
All values are expressed as means ± SD. Statistical tests were performed using a commercial software package (Statistica 8.0, StatSoft). The Kolmogorov–Smirnov test was applied to determine whether the continuous variables were normally distributed. For with-in-group comparison, variables showing normal distributions were evaluated using the paired t test, while variables showing non-normal distributions were evaluated using the Wilcoxon signed rank test. A p value of less than 0.05 was considered statistically significant.
Results
The main baseline characteristics of subjects with metabolic syndrome (MS) are shown in Table 1, along with the characteristics of the control group. The effects of A. melanocarpa extract supplementation after one (1 m) and 2 months (2 m) of therapy are also presented in regards to plasma lipid levels, body mass index (BMI), and waist circumference. No significant adverse effects were recorded, and all patients completed the study. All laboratory safety measurements remained within normal limits.
The mean values of total cholesterol (TC), LDL-C, TG, BMI, and waist circumference were significantly higher in subjects with MS than in the control group p < 0.001. HDL-C was significantly lower in subjects with MS when compared with the control group. Supplementation with AM extract for one or 2 months resulted in significant decreases in TC, LDL-C, and TG; however, these values were still markedly higher in subjects with MS when compared with the control group (p < 0.001). During the study period, no changes in HDL-C, BMI, and waist circumference were observed in subjects with MS.
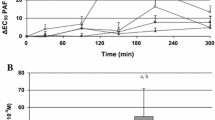
Table 2 compares the average values of aggregation kinetic parameters and the number of platelets in patients suffering from metabolic syndrome as well as in the control group. There were no statistically essential differences between these groups. After 1 month of supplementation with AM extract, we observed a statistically significant inhibition of platelet aggregation. The inhibition of platelet aggregation was manifested by a reduction in maximal aggregation (A max), a diminished initial velocity of the process (v o), and an increase in the time after which A max was reached. However, after 2 months of supplementation, the average values of the aggregation parameters were comparable with those from before supplementation.
We observed no statistically significant differences between the groups (after 1 and 2 months of supplementation) in regards to alterations in shape (PSCmax) and in regards to the number of platelets (PLT).
The kinetic parameter values for the processes of clot formation and fibrinolysis, as well as for coagulation itself, are presented in Tables 3 and 4, respectively. In patients with MS, the overall potential for clot formation and lysis (CLAUC), as well as coagulation (C AUC), were both initially elevated in comparison with the control group. Furthermore, in regards to the evaluation of CLAUC, the time of coagulation (Tf) was lengthened and the initial velocity (Fvo) of plasma coagulation was decreased under the influence of exogenous thrombin and the duration of the transitional phase (Tc). However, the time of coagulation induced by endogenic thrombin was shortened (Tf).
After 1 month of AM supplementation, we observed a statistically significant decrease in the overall potential for clot formation and fibrinolysis (CLAUC), as well as in all of the constituents under the curve of this process (Sf, Sc, and Sl). Furthermore, we estimated that the maximum of coagulation (F max) and fibrinolysis (L max) was also both reduced. Moreover, the times of these processes (Tf and Tl) changed in a statistically significant manner. After 2 months, we did not observe any statistically significant differences between the determined kinetic parameters of clot formation and fibrinolysis.
In regards to coagulation induced by endogenic thrombin, both 1 and 2 months of supplementation induced a statistically significant decrease in the overall potential of coagulation (C AUC), reaching values normally found among healthy individuals.
Throughout the study period, the concentration of fibrinogen among patients with MS was statistically higher than among healthy individuals. One month of AM extract supplementation did not influence the concentration of fibrinogen. However, after 2 months of supplementation, we observed a statistically significant increase in fibrinogen concentration when compared to initial values.
Discussion
Aronia and other berries have been extensively investigated since it was discovered that natural polyphenols, including anthocyanins from various berries, exhibit several health-promoting properties [13].
The cardioprotective activity of AM and AM extracts can be attributed to the effects of anthocyanins. Anthocyanins are lipid-lowering, anti-aggregative, and also exert direct vasoactive action. It should also be mentioned that AM fruits contain significant amounts of niacin, well recognized for its lipid-lowering activity [5]. The lipid-lowering activity of AM preparations has been documented with the use of rat models with artificially induced hypercholesterolemia [14]. It has also been observed that regularly drinking (>6 weeks) AM juice has a beneficial effect on lipid concentrations. Furthermore, a significant decrease in TC, LDL-C, and TG was observed in patients with mild hypercholesterolemia who were not undergoing pharmacological treatment (along with an increase in HDL2) [15].
In a double-blind placebo-controlled parallel trial, AM extract was administered in combination with statins to patients after myocardial infarction. Regardless of the statins, the polyphenol-rich extract significantly reduced the LDL-C oxidation status, blood pressure, and inflammatory marker levels (8-isoprostans, hs-CRP, MCP-1) [16].
During our previous studies on the influence of AM extract, we observed the advantageous decrease in blood pressure, lipid and endothelin-1 concentrations, and levels of oxidative stress markers in 25 patients suffering from MS [17].
Recent experiments have confirmed these aforementioned observations. After supplementation with AM extract for 1 and/or 2 months, we observed statistically significant reductions in the concentrations of TC, LDL-C, and TG among 38 patients with MS. However, during the time of supplementation, alterations in levels of HDL-C were not confirmed.
The anti-platelet activity of polyphenolic AM fruit extract has been confirmed by numerous in vitro studies [18–20]. It should also be noted that the results obtained from in vitro tests significantly differ from those of in vivo studies, as aronia anthocyanins are sensitive to alkaline pancreatic digestion, which substantially modulates their bioavailability [5]. So far, no clinical observations evaluating the influence of aronia on platelet activity and the overall potential for coagulation and lysis have been published. However, there are published studies confirming the beneficial activity of other berries (rich in polyphenols) on platelet function. A preliminary investigation by Wilson et al. [21] evaluated the ability of cranberry juice to inhibit platelet aggregation in vivo. After 4 days of consuming cranberry juice four times a day, ADP- and collagen-induced platelet aggregation declined significantly compared to baseline values. These aggregation responses returned to near baseline levels 4 days after cessation of cranberry juice consumption. Attempts to recreate the inhibition of platelet aggregation in vitro by adding cranberry juice to platelet-rich plasma from subjects who did not consume cranberry juice were not successful [22]. The favorable effects of berry consumption on platelet function were confirmed by a single-blind, randomized, placebo-controlled intervention trial. Seventy-two volunteers consumed 2 portions of berries daily for 8 weeks. Berry consumption significantly inhibited platelet function, measured with a PFA-100 analyzer using a membrane coated with ADP and collagen (CADP-CT). The occlusion time in the CADP-CT system was prolonged by 11% in the berry consuming group. The intervention had no significant effects when epinephrine and collagen were used as platelet activators. Likewise, other plasma biomarkers of platelet activation, coagulation, and fibrinolysis did not change during the intervention [23].
Our studies showed that 1 month of AM extract supplementation significantly inhibited ADP-induced platelet aggregation. We observed not only a 30% decrease in aggregation (A max), but also slowing of this process by over 35%, resulting in the lengthening of the time required to reach the aggregation maximum by about 20%. This observed effect may have some positive implications for the prevention of coronary incidents related to platelet hyperactivity frequently observed among patients with MS [4]. After 2 months of treatment with AM extract, average values of kinetic parameters of aggregation were only slightly lower than initial values. Compliance with the study protocol appeared to be proper after 1 and 2 months of supplementation. Supplementation with AM extract did not influence the general number of platelets in the blood of the examined patients. Furthermore, in every patient, the number of platelets in the PRP used for the estimation of aggregation was always (baseline, 1 and 2 m) brought by PPP to the same value. This is of vital importance because the measured kinetic parameters of aggregation closely depend on the number of platelets in the PRP.
The multi-parameter test utilized in this study of coagulation and fibrinolysis was developed for the in vitro screening of drugs [10]. The usefulness of this method in regards to ex vivo studies was confirmed by a study on a group of 298 elderly subjects. In this group, correlations were observed between the measured kinetic parameters and some of the other typical methods of hemostasis evaluation, as well as between factors such as sex, age, smoking, alcohol consumption, obesity, and physical activity [24]. Antovic [25], by means of spectrophotometric measurement of the overall potential for coagulation (OHP) and fibrinolysis (OFP), showed the existence of correlations between these parameters and different concentrations of coagulation factors (II, V, VII, VIII, IX, X, XI) during in vitro tests. The OHP assay was also evaluated in connection with hypercoagulation in normal pregnancy, preeclampsia, some thrombophilias, coronary heart disease, diabetes, stroke, and vascular surgery, as well as with hypocoagulability. The possible usefulness of the assay in monitoring anticoagulant treatment was also revealed [25].
In our studies, we observed statistically higher initial values for the overall potential of clot formation and lysis (CLAUC), overall potential for plasma clotting (C AUC), and concentrations of fibrinogen in patients with MS when compared with the control group. We also observed some interesting differences between the individual kinetic parameters of CLAUC when comparing patients with MS and the control group. In the control group, we observed a statistically faster formation of fibrinogen fibers under the influence of exogenous thrombin (higher value of Fvo and shortened Tf), and a considerably faster beginning to the lysis of the formed clot (shortened Tc value). Despite the fact that we did not observe significant changes in the kinetics of fibrinolysis, we observed that this examined process functions more efficiently in healthy patients. Alterations of this kind might point to an increase in the thrombotic risk of patients with MS.
After 1 month of supplementation with AM extract, we observed a beneficial reduction in the overall potential for clot formation and fibrinolysis (CLAUC). This change was observed for the area under the curve of all three phases of the examined process (Sf, Sc, and Sl). This observation may indicate that AM supplementation resulted in the modification of many hemostasis factors. However, further molecular studies are required to determine whether these effects are the result of the direct influence of AM on the activity of hemostasis factors or whether these pleiotropic effects are the result of the in vivo activity of AM compounds (anti-inflammatory, influencing function of endothelium, anti-aggregatory, and hypolipemic). After 2 months of AM supplementation, the direction of CLAUC, Sf, Sc, and Sl change was still the same and the measured parameters were still 11% lower on average than initial values. However, these differences were not statistically significant. In the case of the overall potential for coagulation (C AUC), a comparable and beneficial influence was observed after 1 and 2 months of AM extract administration. Although kinetic parameters of the first phase of CL-test and the parameters describing the overall potential of coagulation seem the same, it should be noticed that in fact they differ from each other.
Since fibrin formation is induced by high concentrations of exogenous thrombin in the CL-test, the observed alteration to the parameters (e.g., F max, Tf) results from changes to the fibrinogen particle that influences the structure of the formed clot. It has been confirmed in literature that modification of fibrinogen particles and fibrin (for example as a result of glycation) causes the formation of a fibrin net with an abnormal structure [26]. In the evaluation of the overall potential of coagulation, the addition of small amounts of thrombin with calcium chloride does not result in coagulation initiation but rather induces a feedback reaction leading to the generation of endogenous thrombin, resulting in clot formation. By using both of these tests, a more accurate screening test for the generation of endogenic thrombin as well as clot formation and lysis can be obtained.
The current study has some limitations. The small number of subjects and the design of the study (a placebo-treated group was not included) are both limiting factors; however, this study may be treated as a pilot study.
In conclusion, normalization of hemostasis parameters was observed in patients with metabolic syndrome after both 1 and 2 moths of AM extract administration. We found favorable changes in the overall potential of plasma clotting (C AUC), clot formation and lysis (CLAUC), and the lipid profile of subjects after the consumption of A. melanocarpa extract for 1 month. The beneficial effect of supplementation with AM extract was weaker in regards to platelet aggregation.
References
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y (1999) Novel modulator for endothelial adhesion molecules adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476
Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M (2011) Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res 50:261–266
Cefalu WT, Ye JP, Zuberi A, Ribnicky DM, Raskin I, Liu Z, Wang ZQ, Brantley PJ, Howard L, Lefevre M (2008) Botanicals and the metabolic syndrome. Am J Clin Nutr 87:481S–487S
Sikora J, Markowicz M, Mikiciuk-Olasik E (2009) Role of medical properties of Aronia melanocarpa in the prevention of civilization diseases. Bromatol Chem Toksykol 1:10–17
Kokotkiewicz A, Jaremicz Z, Luczkiewicz M (2010) Aronia plants: a review of traditional use, biological activities, and perspectives for modern medicine. J Med Food 13:255–269
Tsuda T (2008) Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem 56:642–646
De Pergola G, Pannacciulli N (2002) Coagulation and fibrinolysis abnormalities in obesity. J Endocrinol Invest 25:899–904
Born GV (1962) Aggregation of blood platelets by adenosine and reversal. Nature 194:927–929
Kostka B, Sikora J, Para J, Krajewska U, Korzycka L (2007) A new nitrate derivative of piperazine: its influence on platelet activity. Blood Coagul Fibrinol 18:151–156
Kostka B, Para J, Sikora J (2007) A multiparameter test of clot formation and fibrinolysis for in vitro drug screening. Blood Coagul Fibrinol 18:611–618
Friedewald WT, Levly RI, Fredricson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Clauss A (1957) Rapid physiological coagulation method for the determination of fibrinogen. Acta Haematol 17:237–246
Vachleva-Kuzmanova SV, Belcheva A (2006) Current knowledge of Aronia melanocarpa as a medical plant. Folia Medica XLVIII:11–17
Wróblewska M, Juśkiewicz J, Frejnagel S, Oszmiański J, Zduńczyk Z (2008) Physiological influence of chokeberry phenolic in model diet. Acta Aliment Hung 37:589–602
Skoczyńska A, Jędrychowska I, Poręba R, Affelska-Jercha A, Turczyn B, Wojakowska A, Andrzejak R (2007) Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol Rep 59:177–182
Naruszewicz M, Łaniewska I, Millo B, Dłużniewski M (2007) Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infarction (MI). Atherosclerosis 194:e179–e184
Broncel M, Koziróg M, Duchnowicz P, Koter-Michalak M, Sikora J, Chojnowska-Jezierska J (2010) Aronia melanocarpa extract reduces blood pressure,serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med Sci Monit 16:CR28–CR34
Olas B, Wachowicz B, Tomczak A, Erler J, Stochmal A, Oleszek W (2008) Comparative anti-platelet and antioxidant properties of polyphenol-rich extracts from: berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets 19:70–77
Kedzierska M, Olas B, Wachowicz B, Stochmal A, Oleszek WA, Erler J (2011) Changes of platelet antioxidative enzymes during oxidative stress: the protective effect of polyphenol-rich extract from berries of Aronia melanocarpa and grape seeds. Platelets Feb 7 (Epub ahead of print)
Luzak B, Golanski J, Rozalski M, Krajewska U, Olas B, Watala C (2010) Extract from Aronia melanocarpa fruits potentiates the inhibition of platelet aggregation in the presence of endothelial cells. Arch Med Sci 6:141–144
Wilson T, Marley JC (2001) Effects of cranberry juice consumption on platelet aggregation. FASEB J 15:A286
McKay DL, Blumberg JB (2007) Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr Rev 65:490–502
Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A (2008) Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87:323–331
Para J (2007) Evaluation of selected parameters of haemostasis in a random sample of elderly inhabitants of Lodz city. Dissertation, Medical University of Lodz
Antovic A (2010) The overall hemostasis potential: a laboratory tool for the investigation of global hemostasis. Semin Thromb Hemost 36:772–779
Jabłońska-Trypuć A (2007) Molecular mechanism of non-enzymic glication of proteins and meaning. Przegl Kardiodiabetol 2:253–258
Acknowledgments
This study was supported by project N N405 062934 of the Polish Ministry of Science and Higher Education.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sikora, J., Broncel, M., Markowicz, M. et al. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur J Nutr 51, 549–556 (2012). https://doi.org/10.1007/s00394-011-0238-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0238-8