Abstract
Objective
We retrospectively determined factors predicting biologic treatment discontinuation or tapering in patients with axSpA.
Materials and methods
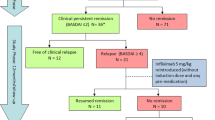
We included 63 nonradiographic axSpA (nr-axSpA) and 138 radiographic axSpA (r-axSpA) patients on biologic treatments for at least 1 year. The biologic dosing intervals were increased in patients who had been in remission for at least 6 months. In patients whose biologic dosing intervals could be increased by 100% for at least 6 months, the agents were stopped at the end of that time. In patients for whom the biologic agents were stopped or tapered, relapse was defined as a Bath Ankylosing Spondylitis Disease activity index score > 4 and a CRP level > 10 mg/L.
Results
The median duration of biologic treatment (all patients) was 2 (1–11) years. Logistic regression analysis did not identify any independent predictor of treatment discontinuation. NSAID use was the only independent predictor of tapering (p = 0.001). The time to relapse after tapering was shorter in patients with r‑axSpA than nr-axSpA (25.97 vs. 39.53 months; p = 0.05). The time to relapse in patients with r‑axSpA was considerably shorter than that in patients with nr-axSpA (5.14 vs. 13 months; p = 0.001). All r‑axSpA patients relapsed over the follow-up period; only 2 nr-axSpA patients did not relapse.
Conclusion
The most significant independent predictor of relapse was NSAID use during treatment. For axSpA patients in remission, tapering of the biologic dosing intervals is more appropriate than discontinuation.
Zusammenfassung
Ziel
Retrospektiv wurden Faktoren untersucht, welche das Absetzen oder Ausschleichen einer Therapie mit Biologika bei Patienten mit axialer Spondylarthritis (axSpA) vorhersagten.
Material und Methoden
Es wurden 63 Patienten mit nichtradiologischer axSpA (nr-axSpA) und 138 mit radiologischer axSpA (r-axSpA) in die Studie einbezogen, welche seit mindestens einem Jahr unter Therapie mit Biologika standen. Die Dosierungsabstände der Biologika wurden bei Patienten, die seit wenigstens 6 Monaten in Remission waren, erhöht. Bei Patienten, deren Dosierungsabstände der Biologika um 100% für mindestens 6 Monate erhöht werden konnten, wurden die Präparate am Ende dieses Zeitraums abgesetzt. Für die Patienten, bei denen die Biologikatherapie abgesetzt oder ausgeschlichen worden war, wurde ein Rezidiv definiert als ein Wert > 4 im Bath Ankylosing Spondylitis Disease Activity Index und ein Spiegel > 10 mg/l für C‑reaktives Protein (CRP).
Ergebnisse
Die mediane Dauer der Biologikabehandlung (für sämtliche Patienten) betrug 2 (1–11) Jahre. Mit der logistischen Regressionsanalyse wurde kein unabhängiger Prädiktor für die Therapiebeendigung identifiziert. Der Gebrauch von nichtsteroidalen Antirheumatika (NSAID) war der einzige unabhängige Prädiktor für das Ausschleichen (p = 0,001). Die Dauer bis zum Rezidiv nach Ausschleichen war bei Patienten mit r‑axSpA kürzer als bei Patienten mit nr-axSpA (25,97 vs. 39,53 Monate; p = 0,05). Die Dauer bis zum Rezidiv bei Patienten mit r‑axSpA war deutlich kürzer als bei Patienten mit nr-axSpA (5,14 vs. 13 Monate; p = 0,001). Bei allen r‑axSpA-Patienten kam es im Laufe der Nachbeobachtungsphase zum Rezidiv; nur 2 nr-axSpA-Patienten blieben rezidivfrei.
Schlussfolgerung
Der wichtigste unabhängige Prädiktor für ein Rezidiv war der Gebrauch von NSAID (nonsteroidal anti-inflammatory drugs) während der Behandlung. Für axSpA-Patienten in Remission ist hinsichtlich der Dosierungsabstände der Biologika das Ausschleichen besser geeignet als das Absetzen.

Similar content being viewed by others
References
Garg N, van den Bosch F, Deodhar A (2014) The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol 28:663–672
Rudwaleit M, van der Heijde D, Landewé R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68(6):777–783
Protopopov M, Poddubnyy D (2018) Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 14:525–533
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
van der Heijde D, Ramiro S, Landewe R et al (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76:978–991
Ward MM, Deodhar A, Gensler LS et al (2019) 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 71:1599–1613
Kroon FP, van der Burg LR, Ramiro S et al (2015) Non-steroidal antiinflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010952.pub2
Ward MM, Deodhar A, Akl EA et al (2016) American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 68:282–298
Furst DE, Louie JS (2019) Targeting inflammatory pathways in axial spondyloarthritis. Arthritis Res Ther 21:135
McGonagle DG, McInnes IB, Kirkham BW et al (2019) The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 78:1167–1178
Arends S, Brouwer E, van der Veer E et al (2011) Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 13(3):R94
Busquets N, Tomero E, Descalzo MÁ et al (2011) BIOBADASER 2.0 Study Group. Age at treatment predicts reason for discontinuation of TNF antagonists: data from the BIOBADASER 2.0 registry. Rheumatology 50(11):1999–2004
Malaviya AN, Rawat R, Agrawal N et al (2017) The Nonradiographic axial spondyloarthritis, the radiographic axial spondyloarthritis, and ankylosing spondylitis: the Tangled Skein of rheumatology. Int J Rheumatol 2017:1–9
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291
Jenkinson TR, Mallorie PA, Whitelock HC et al (1994) Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 21(12):1694–1698
Baraliakos X, Listing J, Brandt J et al (2005) Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 7(3):R439–R444
Chen X, Zhang T, Wang W et al (2018) Analysis of relapse rates and risk factors of tapering or stopping pharmacologic therapies in axial spondyloarthritis patients with sustained remission. Clin Rheumatol 37(6):1625–1632
Mease PJ, van der Heijde D, Karki C et al (2018) Tumor necrosis factor inhibitor discontinuation in patients with ankylosing spondylitis: an observational study from the US-based Corrona registry. Rheumatol Ther 5(2):537–550
Lubrano E, Perrotta MF, Manara M et al (2016) Predictors of loss of remission and disease flares in patients with axial spondyloarthritis receiving antitumor necrosis factor treatment: a retrospective study. J Rheumatol 43(8):1541–1546. https://doi.org/10.3899/jrheum.160363 (Erratum in: J Rheumatol. 2016 Sep;43(9):1772)
Michielsens CAJ, den Broeder N, Mulder MLM et al (2021) Tumour necrosis factor inhibitor dose adaptation in psoriatic arthritis and axial spondyloarthritis (TAPAS): a retrospective cohort study. Rheumatology. https://doi.org/10.1093/rheumatology/keab741
Wetterslev M, Georgiadis S, Juul Sørensen I et al (2021) Tapering of TNF inhibitors in axial spondyloarthritis in routine care-2-year clinical and MRI outcomes and predictors of successful tapering. Rheumatology. https://doi.org/10.1093/rheumatology/keab755
Braun J, Baraliakos X, Deodhar A et al (2019) Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4‑year results from the MEASURE 1 study. Rheumatology 58:859–868
Braun J, Kiltz U, Bühring B et al (2021) Secukinumab in axial spondyloarthritis: a narrative review of clinical evidence. Ther Adv Musculoskel Dis 13:1–18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Harman and N. Kaban declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Redaktion
Ulf Müller-Ladner, Bad Nauheim
Uwe Lange, Bad Nauheim

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Harman, H., Kaban, N. Is tapering or discontinuation of biologic treatment in patients with radiographic and nonradiographic axial spondyloarthritis reasonable?. Z Rheumatol 83 (Suppl 1), 55–61 (2024). https://doi.org/10.1007/s00393-022-01226-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-022-01226-0