Abstract
Background
The 2022 AHA/ACC/HFSA guidelines for the management of heart failure (HF) makes therapeutic recommendations based on HF status. We investigated whether the prognosis of in-hospital cardiac arrest (IHCA) could be stratified by HF stage and left ventricular ejection fraction (LVEF).
Methods
This single-center retrospective study analyzed the data of patients who experienced IHCA between 2005 and 2020. Based on admission diagnosis, past medical records, and pre-arrest echocardiography, patients were classified into general IHCA, at-risk for HF, pre-HF, HF with preserved ejection fraction (HFpEF), and HF with mildly reduced ejection fraction or HF with reduced ejection fraction (HFmrEF-or-HFrEF) groups.
Results
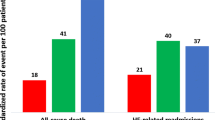
This study included 2,466 patients, including 485 (19.7%), 546 (22.1%), 863 (35.0%), 342 (13.9%), and 230 (9.3%) patients with general IHCA, at-risk for HF, pre-HF, HFpEF, and HFmrEF-or-HFrEF, respectively. A total of 405 (16.4%) patients survived to hospital discharge, with 228 (9.2%) patients achieving favorable neurological recovery. Multivariable logistic regression analysis indicated that pre-HF and HFpEF were associated with better neurological (pre-HF, OR: 2.11, 95% confidence interval [CI]: 1.23–3.61, p = 0.006; HFpEF, OR: 1.90, 95% CI: 1.00–3.61, p = 0.05) and survival outcomes (pre-HF, OR: 2.00, 95% CI: 1.34–2.97, p < 0.001; HFpEF, OR: 1.91, 95% CI: 1.20–3.05, p = 0.007), compared with general IHCA.
Conclusion
HF stage and LVEF could stratify patients with IHCA into different prognoses. Pre-HF and HFpEF were significantly associated with favorable neurological and survival outcomes after IHCA. Further studies are warranted to investigate whether HF status-directed management could improve IHCA outcomes.
Similar content being viewed by others
Introduction
An estimated 292,000 patients in the United States experience in-hospital cardiac arrest (IHCA) each year [1]. Approximately 18.8% of patients with IHCA survive to hospital discharge, and only about 12.9% of these patients have favorable neurological recovery at hospital discharge [1].
Heart failure (HF) is a major public health issue, leading to over 1 million hospitalizations annually in the United States [2]. HF is present in approximately 20–30% of patients with IHCA [3,4,5], accounting for 12.6% of the etiologies of IHCA [6]. However, only a few studies [5, 7,8,9,10] have investigated the prognosis of HF complicated by IHCA. Also, the findings from these studies [5, 7,8,9,10] have been inconsistent and contradictory.
HF is a complex syndrome resulting from various functional or structural abnormalities impairing ventricular filling or blood pumping [11, 12]. The 2022 American Heart Association/American College of Cardiology/ Heart Failure Society of America (AHA/ACC/HFSA) Guidelines [11, 12] identify four stages to describe the trajectory of HF as a progressive disease. These stages include at-risk for HF (stage A), pre-HF (stage B), symptomatic HF (stage C), and advanced HF (stage D). In addition to HF stages, left ventricular ejection fraction (LVEF) is essential for HF management [11, 12].
The 2022 AHA/ACC/HFSA Guidelines [11, 12] make therapeutic recommendations based on HF stages and LVEF. Nevertheless, these recommendations focus on long-term management [11,12,13]. In the current study, we aimed to investigate the prognosis of IHCA stratified by HF stages and LVEF, which may facilitate the identification of patient groups susceptible to poor IHCA outcomes and tailoring of personalized management for patients with IHCA.
Materials and Methods
This study was conducted in compliance with the Declaration of Helsinki amendments. The Research Ethics Committee of National Taiwan University Hospital (NTUH) approved this study (reference number: 201804089RINC) and waived the informed consent requirement. The study was conducted and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14].
Study design and setting
This observational study was a secondary analysis using prospectively collected IHCA databases registered in NTUH. NTUH is a tertiary medical center with 2,600 beds, including 220 intensive care unit (ICU) beds. The Centre of Quality Management at NTUH receives reports of all IHCA events and prospectively maintains an IHCA registry, aiming to improve patient safety systematically. When a patient experiences cardiac arrest in the general wards, a code team is activated per hospital policy. If cardiac arrest occurs in an ICU, resuscitation is performed by the staff in the unit without activating the code team. Cardiopulmonary resuscitation (CPR) is performed for all patients based on up-to-date resuscitation guidelines [15, 16].
Patient inclusion criteria
For this study, the data of patients who experienced IHCA from 2005 to 2020 were reviewed and included, if they met the following criteria: (1) patients with age ≥ 18, (2) patients with a documented absence of pulse and record of receiving chest compressions for at least 2 min, and (3) patients without a do-not-resuscitate order prior to the arrest. Patients admitted for major trauma were excluded. In cases where a patient experienced multiple cardiac arrest events during their hospitalization, only the first event was retrieved for analysis.
Data collection and variable definitions
Data regarding IHCA events were prospectively collected from the electronic medical records of NTUH. Patient comorbidities were defined according to Get With The Guidelines-Resuscitation registry [10, 17]. Peri-CPR variables were derived from the Utstein template [17]. In contrast, data used to reclassify patients by HF stages and LVEF for the current study were retrospectively collected, including N-terminal pro-B natriuretic peptide (NT-proBNP) and echocardiographic findings. Both NT-proBNP and echocardiographic findings were retrieved from the index hospitalization or within 3 months before the IHCA event [18]. Echocardiographic findings related to HF staging were abstracted [11, 12].
Grouping by HF status
According to the 2022 AHA/ACC/HFSA Guidelines [11, 12], we classified HF into five groups based on the admission diagnosis, prior medical records, laboratory studies, and echocardiographic findings: (1) general IHCA, (2) at-risk for HF, (3) pre-HF, (4) HF with preserved ejection fraction (HFpEF), and (5) HF with mildly reduced EF or HF with reduced ejection fraction (HFmrEF-or-HFrEF). At-risk for HF was defined as the absence of HF in the admission diagnosis and prior medical records and the presence of hypertension, cardiovascular diseases, diabetes, or obesity [19]. Pre-HF was defined as the absence of HF in the admission diagnosis and prior medical records and the presence of structural heart disease, evidence of increased filling pressures, or elevated NT-proBNP level. The thresholds for echocardiographic parameters and NT-proBNP level to define pre-HF were consistent with the 2022 AHA/ACC/HFSA Guidelines [11, 12]. HFpEF, HFmrEF, and HFrEF were defined as the presence of HF in the admission diagnosis and/or prior medical records with LVEF \(\ge\) 50%, between 41–49%, or ≤ 40% [11, 12], respectively. Patients who did not meet the above criteria were categorized as the general IHCA group.
Study outcomes
The primary and secondary outcomes were favorable neurological status and survival at hospital discharge, respectively. Favorable neurological status was defined as a score of 1 or 2 on the Cerebral Performance Category (CPC) scale [20], which was retrospectively recorded by research assistants blinded to the research hypothesis.
Statistical Analysis
Data were analyzed using R version 4.4.2 software (R Foundation for Statistical Computing, Vienna, Austria). Categorical data are expressed as counts and proportions, and continuous data are expressed as medians and interquartile ranges (IQRs). Categorical variables were compared using the chi-squared test, whereas continuous variables were compared using the Kruskal–Wallis test. A two-tailed p-value < 0.05 was considered statistically significant.
The odds ratio (OR) was selected as the outcome measure. Multivariable logistic regression analyses were used to examine the associations between independent variables and outcomes. All available independent variables, including HF status, age, sex, comorbidities, peri-CPR variables, and critical care interventions, were considered in the regression model, regardless of whether they were determined to be significant by univariate analyses. The stepwise variable selection procedure was adopted. Generalized additive models (GAMs) [21] were used to examine the nonlinear effects of the continuous variables and identify the appropriate cut-off point(s) for dichotomizing a continuous variable.
In the main analysis, the association between each HF status and outcomes was compared with that of general IHCA. Subgroup analysis was performed to examine the association between each HF status and IHCA period, diabetes, or initial arrest rhythms to identify the factors accounting for the differences in prognosis among the different HF categorizations. The IHCA period was split into three intervals based on the 2010 [22, 23] and 2015 [24, 25] guidelines for CPR. In the sensitivity analysis, only patients with available echocardiographic data were included. Echocardiographic parameters and NT-proBNP level were substituted for HF status in the regression analysis to examine the association between individual echocardiographic parameters with outcomes.
Results
As shown in Figs. 1 and 2, 466 patients were included in the main analysis. Among these patients, 1,159 (47.0%) patients had echocardiographic data and were included in the sensitivity analysis.
In the main analysis (Table 1), 485 (19.7%), 546 (22.1%), 863 (35.0%), 342 (13.9%), and 230 (9.3%) patients were categorized as patients with general IHCA, at-risk for HF, pre-HF, HFpEF, and HFmrEF-or-HFrEF, respectively. Compared with patients with general IHCA, patients with at-risk of HF, pre-HF, HFpEF, or HFmrEF-or-HFrEF were older, had more comorbidity burdens except for metastatic cancer or any blood-borne malignancy, experienced higher proportions of initial shockable rhythms, and underwent more extracorporeal membrane oxygenation (ECMO) and percutaneous coronary intervention (PCI) procedures. A total of 405 (16.4%) patients survived to hospital discharge, with 228 (9.2%) patients achieving favorable neurological recovery. Patients with pre-HF, HFpEF, and HFmrEF-or-HFrEF had similar proportions of favorable neurological and survival outcomes, all of which were higher than that of general IHCA.
In the main analysis, all independent variables were considered in the regression model for variable selection. After confounding factors were accounted for, the results (Table 2) indicated that pre-HF and HFpEF were associated with favorable neurological (pre-HF, OR: 2.11, 95% confidence interval [CI]: 1.23–3.61, p = 0.006; HFpEF, OR: 1.90, 95% CI: 1.00–3.61, p = 0.05) and survival outcomes (pre-HF, OR: 2.00, 95% CI: 1.34–2.97, p < 0.001; HFpEF, OR: 1.91, 95% CI: 1.20–3.05, p = 0.007).
The subgroup analysis (Figs. 2 and 3, Supplemental Table 1–3) revealed that during 2015–2020, pre-HF (OR: 3.27, 95% CI: 1.08–9.91, p = 0.04) and HFpEF (OR: 3.88, 95% CI: 1.16–12.98, p = 0.03) were associated with favorable neurological recovery. Additionally, for HF status stratified by diabetes or initial arrest rhythms, HFpEF with diabetes (OR: 3.03, 95% CI: 1.38–6.67, p = 0.006) and HFmrEF-or-HFrEF with shockable rhythms (OR: 3.12, 95% CI: 1.33–7.31, p = 0.009) were noted to be associated with favorable neurological outcomes.
Forest plot of adjusted odds ratio of each subgroup for favorable neurological outcome at hospital discharge. Subgroups were stratified by HF status and (A) period of IHCA, (B) diabetes, and (C) initial arrest rhythms. Please refer to Supplemental Table 1–3 for the confounding factors accounted for. HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; IHCA: in-hospital cardiac arrest; OR: odds ratio
Forest plot of adjusted odds ratio of each subgroup for survival at hospital discharge. Subgroups were stratified by HF status and (A) period of IHCA, (B) diabetes, and (C) initial arrest rhythms. Please refer to Supplemental Table 1–3 for the confounding factors accounted for. HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; IHCA: in-hospital cardiac arrest; OR: odds ratio
Supplemental Table 4 shows the characteristics of patients who were included in the sensitivity analysis. The median time between pre-arrest echocardiography and IHCA event was 10.4 days (IQR: 50.9 days). Since not all patients had quantitative LVEF or NT-proBNP values, these variables were transformed into binary variables for analysis. Among all the echocardiographic parameters, only concentric remodeling was associated with favorable neurological recovery (OR: 1.66, 95% CI: 1.03–2.69, p = 0.04) (Supplemental Table 5).
Discussion
Main Findings
First, this 16-year cohort study revealed that pre-HF accounted for the highest proportion of IHCA. Second, the proportions of favorable neurological recovery in patients with pre-HF, HFpEF, or HFmrEF-or-HFrEF were similar, which were higher than that of general IHCA. Third, only pre-HF and HFpEF were significantly associated with better outcomes, which could be due to the presence of several subgroups stratified by HF status and IHCA period, diabetes, or initial shockable rhythms. Finally, among the echocardiographic parameters, only concentric remodeling was significantly associated with better outcomes.
Comparison with previous studies
Hessulf et al. [5] analyzed the data of approximately 18,000 patients with IHCA during 2006–2015 and indicated that HF was significantly associated with lower survival. Using a large AHA registry, Chan et al. [10] analyzed the data of approximately 48,000 patients with IHCA during 2007–2010 and suggested that HF was not associated with survival. Considering LVEF, in a single-center study on 613 patients with IHCA during 2004–2006, Gonzalez et al. [18] suggested that LVEF < 45% was significantly associated with lower survival. Nevertheless, Aune et al. [7] suggested that LVEF < 50% was associated with higher survival by analyzing the data of 6,378 patients with IHCA during 2009–2019. Similarly, Chouairi et al. [8] studied 56,170 HF cases complicated with IHCA and demonstrated that, compared with HFpEF, HFrEF was significantly associated with higher survival after an IHCA event. Therefore, for all analyses of HF as an entire entity or stratified by LVEF, contradictory results were reported by the previous studies [5, 7,8,9,10].
Grouping of patients with HF
In these previous studies [5, 7,8,9,10], patients with pre-HF were unidentified and thus mixed with patients with general IHCA for analysis. Our study revealed that, first, the proportion of pre-HF was the highest among all HF groups (Table 1). Second, approximately 21.9% of patients with pre-HF who had echocardiographic data demonstrated an LVEF < 50% (Supplemental Table 4). Third, pre-HF was associated with better outcomes (Table 2). Hence, the different methods in classifying pre-HF may account for the contradictory results of previous studies [5, 7,8,9,10]. For example, if pre-HF was analyzed as the absence of HF, the results may be biased toward favoring the positive association between the absence of HF and outcomes [5]. In contrast, if patients with pre-HF who had LVEF < 50% were counted as HFrEF, the results may suggest that HFrEF was associated with better outcomes [7, 8].
Besides at-risk for HF and pre-HF, no further classifications of symptomatic and advanced HF were made in our study. Advanced HF is defined as refractory symptoms despite optimal management [11, 12]. For advanced HF, palliative care is an important alternative. Most patients with advanced HF may not receive CPR if experiencing IHCA, leaving most patients with HF in our study classified as symptomatic HF and making further prognosis stratification unlikely. Therefore, LVEF was adopted to classify HF because LVEF is a critical factor in HF management [11, 12].
Table 1 demonstrates the significant differences in comorbidity burdens among these HF groups, with more cases of cardiovascular diseases in the HFmrEF-or-HFrEF group and more cases of cancer in the general IHCA group. These findings may explain the differences in the use of post-resuscitation interventions, such as ECMO and PCI. These significant between-group differences may justify our classification method for HF status.
Main and subgroup analyses
Most previous studies [5, 7,8,9,10] investigated the association between HF and survival after an IHCA. Post–cardiac arrest myocardial dysfunction is an important component of post-cardiac arrest syndrome [26] and manifests as global hypokinesis, leading to reduced cardiac output and hypotension [26]. In a retrospective analysis, Yao et al. [27] indicated that HF was a significant predictor of post-cardiac arrest myocardial dysfunction. Therefore, patients with HF may be more likely to suffer from post-cardiac arrest hypotension caused by myocardial dysfunction. In our previous animal studies, we had noted the presence of prolonged post-cardiac arrest cerebral hypoperfusion [28,29,30], the so-called no-reflow phenomenon [31]. Post-cardiac arrest hypotension may worsen cerebral hypoperfusion, leading to worse neurological recovery [32, 33]. Taken together, patients with HF may have higher risk in developing post-cardiac arrest myocardial dysfunction, which may lead to post-arrest hypotension, aggravate cerebral hypoperfusion and worsen neurological recovery.
Our study was the first to reveal the neuroprognostic effect of different HF status on IHCA outcomes. Our study indicated that pre-HF or HFpEF was not only associated with survival but also with better neurological recovery than general IHCA. For pre-HF, no pharmacotherapy demonstrated definite benefits, except for patients with depressed LVEF [11, 12]. The natriuretic peptide-based screening for early identification of pre-HF was suggested by the 2017 ACC/AHA/HFSA update [34] to prevent the progression of pre-HF to HF. Jia et al. [35] indicated that incorporating biomarkers, such as NT-proBNP, could reclassify approximately 20% of older individuals without diagnosed HF to pre-HF. Interestingly, Table 1 demonstrates that the proportion of pre-HF among all patients with IHCA increased substantially after 2010. Possibly, since 2010, physicians initiated the use of biomarkers in evaluating patients with potential pre-HF more frequently. Identifying pre-HF early offers an opportunity to initiate lifestyle modification and better control of comorbidities, which may explain the better survival after 2010 and better neurological outcomes after 2015 for patients with pre-HF.
Similar to pre-HF, until now, HFpEF had no definitive benefits from therapy, except for sodium-glucose cotransporter-2 inhibitors [11, 12]. Nonetheless, during the study period, use of sodium-glucose cotransporter-2 inhibitors for HFpEF had not yet been approved in Taiwan. Among patients with HFpEF, 44.7% had diabetes (Table 1). Interestingly, the subgroup analysis revealed that patients with both HFpEF and diabetes were associated with favorable neurological recovery (Fig. 2). Jin et al. [36] indicated that in patients with diabetes, administration of metformin within 24 h before IHCA was associated with better neurological and survival outcomes, probably due to the cardioprotective [37] and neuroprotective effects [38,39,40] of metformin. According to the current American Diabetes Association guidelines [41], the effect of metformin on HF is considered neutral, and metformin is considered to be the first-line antidiabetic medication in all patients with type 2 diabetes, including those with HF. Therefore, most patients with HFpEF were possibly prescribed metformin in our study, leading to better outcomes.
Additionally, the subgroup analysis demonstrated that for patients with HFmrEF-or-HFrEF, the presence of shockable rhythms was associated with better outcomes (Figs. 2 and 3). For patients with HFmrEF-or-HFrEF, initial shockable rhythms may suggest the presence of a correctable cardiac cause, justifying the aggressive management of IHCA. In contrast, initial non-shockable rhythms may suggest that IHCA was caused by pump failure or by other non-cardiac causes, with HFmrEF-or-HFrEF being a significant comorbidity, impeding the success of the resuscitation efforts.
Taken together, the subgroup analysis helped explain some differences in prognosis among HF statuses. Nonetheless, due to reduced patient numbers in each subgroup, the results of subgroup analysis could only be considered hypothesis-generating or explanatory rather than definitive results.
Sensitivity analysis
Few studies analyzed the pre-arrest echocardiographic data for IHCA. Using echocardiographic data measured within 3 months before IHCA, Gonzalez et al. [18] indicated that LVEF < 45% was associated with lower survival. Our sensitivity analysis indicated that only concentric remodeling was associated with better outcomes, whereas LVEF < 50% was not. Supplemental Table 4 shows that among patients with LVEF < 50%, 43.6% (157/360) were classified as patients with pre-HF. Since pre-HF was associated with better outcomes, the potential association between LVEF < 50% and worse outcomes may thus become less significant. Similarly, for patients with concentric remodeling, approximately 82.1% (230/280) were classified as patients with pre-HF, which may explain the significant association between concentric remodeling and favorable outcomes. These results suggest that, compared with individual echocardiographic findings, comprehensive HF status evaluated by HF stages and LVEF may be more prognostic of IHCA outcomes. This observation was consistent with that of a previous study [42], which demonstrated that isolated structural or functional abnormalities may not be associated with an increased risk of HF hospitalization or death. Nonetheless, due to the limitation of the retrospective study, most patients with general IHCA and at-risk for HF did not have recent echocardiographic data for comparison and were excluded from sensitivity analysis. This selection bias may distort the association between echocardiographic findings and IHCA outcomes.
Future applications
The 2022 AHA/ACC/HFSA Guidelines for HF [11, 12] emphasized the importance of guideline-directed medical therapy delivered according to different HF statuses. Although CPR guidelines recommend that echocardiography should be performed early after the return of spontaneous circulation [15, 43], the application of these echocardiographic data to optimize post-CPR management remains unclear. Our study revealed that the prognosis of IHCA could be stratified by HF stages and LVEF. Future studies are warranted to investigate whether HF status-directed management, such as selection of hemodynamic goal, adequate administration of inotropic medications, and rehabilitation plans, could improve short- and long-term IHCA outcomes.
Study Limitations
First, this study was a retrospective observational study, which could only establish an association between independent and dependent variables rather than a causal relationship. The current analysis did not consider critical confounding factors, such as administered medications. Second, instead of HF symptoms, the classification of HF groups was based on admission diagnosis or past medical records. Therefore, only patients with HF symptoms severe enough to be hospitalized would be categorized as HFpEF or HFmrEF-or-HFrEF. Otherwise, they would most likely be classified as pre-HF. Since symptoms were subjective and challenging to be studied retrospectively, further prospective studies are warranted. Third, HFmrEF was not separated from HFrEF because patients with HFmrEF usually remain in a dynamic trajectory, staggering between HFrEF and HFrEF. Also, no specific therapeutic recommendations were available exclusively for HFmrEF. Hence, patients with HFmrEF and HFrEF were combined to form one study group. Fourth, in comparison with 2022 AHA/ACC/HFSA Guidelines [11, 12], we did not use metabolic syndrome, exposure to cardiotoxic agents, genetic variant for cardiomyopathy, or positive family history of cardiomyopathy to define patients at risk for HF because these items were not required variables in the Get With The Guidelines-Resuscitation registry [10, 17]. Table 2 shows that there were no significant differences in neurological or survival outcomes between general IHCA and at-risk for HF. Misclassification of at-risk for HF may have occurred and caused the null results, which should be further examined in future studies.
Conclusions
HF stage and LVEF could stratify patients with IHCA into different prognostic outcome groups. Pre-HF and HFpEF were significantly associated with favorable neurological recovery and survival after IHCA. Further studies are warranted to investigate whether HF status-directed management could improve IHCA outcomes.
Data Availability
Data are available from the corresponding author upon reasonable request.
References
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE et al (2023) Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 147(8):e93–e621
Clark KA, Reinhardt SW, Chouairi F, Miller PE, Kay B, Fuery M, Guha A, Ahmad T, Desai NR (2022) Trends in Heart Failure Hospitalizations in the US from 2008 to 2018. J Cardiac Fail 28(2):171–180
Carnicelli AP, Keane R, Brown KM, Loriaux DB, Kendsersky P, Alviar CL, Arps K, Berg DD, Bohula EA, Burke JA et al (2023) Characteristics, therapies, and outcomes of In-Hospital vs Out-of-Hospital cardiac arrest in patients presenting to cardiac intensive care units: From the critical care Cardiology trials network (CCCTN). Resuscitation 183:109664
Chan PS, Kennedy KF, Girotra S (2023) Updating the model for Risk-Standardizing survival for In-Hospital cardiac arrest to facilitate hospital comparisons. Resuscitation 183:109686
Hessulf F, Karlsson T, Lundgren P, Aune S, Strömsöe A, Södersved Källestedt ML, Djärv T, Herlitz J, Engdahl J (2018) Factors of importance to 30-day survival after in-hospital cardiac arrest in Sweden - A population-based register study of more than 18,000 cases. Int J Cardiol 255:237–242
Allencherril J, Lee PYK, Khan K, Loya A, Pally A (2022) Etiologies of In-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation 175:88–95
Aune E, McMurray J, Lundgren P, Sattar N, Israelsson J, Nordberg P, Herlitz J, Rawshani A (2022) Clinical characteristics and survival in patients with heart failure experiencing in hospital cardiac arrest. Sci Rep 12(1):5685
Chouairi F, Miller PE, Loriaux DB, Katz JN, Sen S, Ahmad T, Fudim M (2023) Trends and Outcomes in Cardiac Arrest Among Heart Failure Admissions. Am J Cardiol 194:93–101
Hooks M, Downey MC, Joppa S, Beard A, Gravely A, Tholakanahalli V, Adabag S (2021) Arrhythmic causes of in-hospital cardiac arrest among patients with heart failure with preserved ejection fraction. Heart Rhythm O2 2(6Part A):665–667
Chan PS, Berg RA, Spertus JA, Schwamm LH, Bhatt DL, Fonarow GC, Heidenreich PA, Nallamothu BK, Tang F, Merchant RM (2013) Risk-standardizing survival for in-hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol 62(7):601–609
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR et al (2022) 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145(18):e876–e894
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR et al (2022) 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145(18):e895–e1032
Chiang CE, Hung CL, Wu YW, Lin TH, Ueng KC, Sung SH, Wu CK, Chao TH, Lin HJ, Lin YH et al (2023) 2023 Consensus of Taiwan Society of Cardiology on the Pharmacological Treatment of Chronic Heart Failure. Acta Cardiol Sin 39(3):361–390
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147(8):573–577
Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT et al (2020) Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 142(16_Suppl_2):S366-s468
Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T et al (2021) European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 161:115–151
Nolan JP, Berg RA, Andersen LW, Bhanji F, Chan PS, Donnino MW, Lim SH, Ma MH, Nadkarni VM, Starks MA et al (2019) Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A Consensus Report From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation 140(18):e746–e757
Gonzalez MM, Berg RA, Nadkarni VM, Vianna CB, Kern KB, Timerman S, Ramires JA (2008) Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation 117(14):1864–1872
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163. https://doi.org/10.1016/S0140-6736(03)15268-3
Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, Nadkarni VM, Abella BS, Adrie C, Berg RA et al (2011) Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation 124(19):2158–2177
Hastie TJ, Tibshirani RJ (1990) Generalized Additive Models. Chapman & Hall, London and New York
Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM et al (2010) Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122(18 Suppl 3):S729-767
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, Perkins GD (2010) European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult Adv Life Support Resuscitation 81(10):1305–1352
Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O’Neil BJ, Paxton JH, Silvers SM et al (2015) Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132(18 Suppl 2):S444-464
Soar J, Nolan JP, Böttiger BW, Perkins GD, Lott C, Carli P, Pellis T, Sandroni C, Skrifvars MB, Smith GB (2015) European resuscitation council guidelines for resuscitation 2015: section 3. Adult Adv Life Support Resuscitation 95:100–147
Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC et al (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118(23):2452–2483
Yao Y, Johnson NJ, Perman SM, Ramjee V, Grossestreuer AV, Gaieski DF (2018) Myocardial dysfunction after out-of-hospital cardiac arrest: predictors and prognostic implications. Intern Emerg Med 13(5):765–772
Wang CH, Chang WT, Huang CH, Tsai MS, Liu SH, Chen WJ (2020) Cerebral Blood Flow-Guided Manipulation of Arterial Blood Pressure Attenuates Hippocampal Apoptosis After Asphyxia-Induced Cardiac Arrest in Rats. J Am Heart Assoc 9(13):e016513
Wang CH, Huang CH, Tsai MS, Wang CC, Chang WT, Liu SH, Chen WJ (2022) Inhaled Carbon Dioxide Improves Neurological Outcomes by Downregulating Hippocampal Autophagy and Apoptosis in an Asphyxia-Induced Cardiac Arrest and Resuscitation Rat Model. J Am Heart Assoc 11(21):e027685
Wang CH, Chang WT, Huang CH, Tsai MS, Wang CC, Liu SH, Chen WJ (2023) Optimal inhaled oxygen and carbon dioxide concentrations for post-cardiac arrest cerebral reoxygenation and neurological recovery. iScience 26(12):108476
Ames A 3rd, Wright RL, Kowada M, Thurston JM, Majno G (1968) Cerebral ischemia. II. The no-reflow phenomenon. The Am J Pathol 52(2):437–453
Bhate TD, McDonald B, Sekhon MS, Griesdale DE (2015) Association between blood pressure and outcomes in patients after cardiac arrest: a systematic review. Resuscitation 97:1–6
Wang CH, Huang CH, Chang WT, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ (2015) Optimal blood pressure for favorable neurological outcome in adult patients following in-hospital cardiac arrest. Int J Cardiol 195:66–72
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM (2017) 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136(6):e137–e161
Jia X, Al Rifai M, Ndumele CE, Virani SS, de Lemos JA, Lee E, Shah AM, Echouffo-Tcheugui JB, Bozkurt B, Hoogeveen R et al (2023) Reclassification of Pre-Heart Failure Stages Using Cardiac Biomarkers: The ARIC Study. JACC Heart Fail 11(4):440–450
Jin BY, Song J, Kim J, Park JH, Kim SJ, Cho H, Moon S, Kim DH, Ahn S (2023) Association between metformin and survival outcomes in in-hospital cardiac arrest patients with diabetes. J Crit Care 73:154171
Sun D, Yang F (2017) Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys Res Commun 486(2):329–335
Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z (2018) Metformin improves neurologic outcome via amp-activated protein kinase–mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc 7(12):e008389
Chuan L, Zhang L, Fu H, Yang Y, Wang Q, Jiang X, Li Z, Ni K, Ding L (2021) Metformin prevents brain injury after cardiopulmonary resuscitation by inhibiting the endoplasmic reticulum stress response and activating AMPK-mediated autophagy. Scott Med J 66(1):16–22
Chuan L, Huang X, Fan C, Wen S, Yang X, Wang J, Ren J, Ru J, Ding L (2021) Metformin ameliorates brain damage caused by cardiopulmonary resuscitation via targeting endoplasmic reticulum stress-related proteins GRP78 and XBP1. Eur J Pharmacol 891:173716
Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S (2022) 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diab Care 45(Supplement_1):S125–S143
Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska-Newton A, Sueta CA, Mosley TH (2017) Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation 135(3):224–240
Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM et al (2021) European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 161:220–269
Acknowledgements
We thank the staff of the 3rd Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support. We thank Centre of Quality Management of National Taiwan University Hospital for providing the list of patients sustaining in-hospital cardiac arrest.
Funding
Author Chih-Hung Wang received a grant (113-R0006) from the National Taiwan University Hospital. National Taiwan University Hospital had no involvement in designing the study, collecting, analysing or interpreting the data, writing the manuscript, or deciding whether to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Chih-Hung Wang: Conceptualization, Methodology, Validation, Resources, Formal analysis, Investigation, Data curation, Writing – original draft, Project administration; Li-Ting Ho: Validation, Resources, Writing – review & editing, Project administration; Meng-Che Wu: Validation, Resources, Writing – review & editing, Project administration; Cheng-Yi Wu: Validation, Resources, Writing – review & editing, Project administration; Joyce Tay: Validation, Resources, Writing – review & editing, Project administration; Pei-I Su: Validation, Resources, Writing – review & editing, Project administration; Min-Shan Tsai: Validation, Resources, Writing – review & editing, Project administration; Yen-Wen Wu: Validation, Resources, Writing – review & editing, Project administration; Wei-Tien Chang: Writing – review & editing, Supervision; Chien-Hua Huang: Writing – review & editing, Supervision; Wen-Jone Chen: Writing – review & editing, Supervision.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was conducted in accordance with the amendments of the Declaration of Helsinki. The Institutional Review Board approved this study (reference number: 201804089RINC) and waived the requirement for informed consent.
Consent or publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, CH., Ho, LT., Wu, MC. et al. Prognostic implication of heart failure stage and left ventricular ejection fraction for patients with in-hospital cardiac arrest: a 16-year retrospective cohort study. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02403-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02403-8