Abstract
Aims
This study aimed to use intravascular ultrasound (IVUS) data to reveal the mechanism of lesion progression in the native coronary circulation proximal to bypass grafts after coronary artery bypass grafting (CABG).
Methods and results
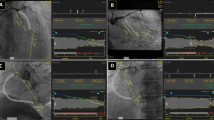
We reviewed IVUS images in 86 patients with an angiographically significant left main coronary artery (LMCA) stenosis. Overall, 41 patients underwent CABG more than 6 months (mean 8.2 ± 6.1 years) previously and had at least one patent graft to the left coronary artery system. The number of patent grafts to the left coronary artery was 1.4 ± 0.7. Comparing patent graft vs. non-CABG groups, external elastic membrane and lumen areas and remodeling index at the minimum lumen area (MLA) site trended smaller with no difference in the plaque & media area. In addition, patients in the patent graft group had more LMCA calcium whether defined by cross-sectional (arc at the MLA site of 141 ± 109° vs. 88 ± 108°, P = 0.025) or longitudinal measurements (calcium length index, calculated as LMCA calcium length divided by total LMCA length, 0.69 ± 0.38 vs. 0.50 ± 0.42, P = 0.035).
Conclusions
Negative remodeling may be the main mechanism of lesion progression proximal to a patent bypass graft, and more calcium was found in LMCA after CABG compared with non-CABG patients.
Similar content being viewed by others
References
Eagle KA, Guyton RA, Davidoff R et al (2004) ACC/AHA 2004 guideline updates for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee to update the 1999 guidelines for coronary artery bypass graft surgery). J Am Coll Cardiol 44:213–310
Smith SC Jr, Feldman TE, Hirshfeld JW Jr et al (2006) ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/SCAI writing committee to update 2001 guidelines for percutaneous coronary intervention). J Am Coll Cardiol 47:216–235
Patel MR, Dehmer GJ, Hirshfeld JW et al (2009) ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology endorsed by the American Society of echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 53:530–553
Rupprecht HJ, Hamm C, Ischinger T et al (1996) On behalf of the GABI study group. Angiographic follow-up results of a randomized study on angioplasty versus bypass surgery (GABI trial). Eur Heart J 17:1192–1198
Guthaner DF, Robert EW, Alderman EL et al (1979) Long-term serial angiographic studies after coronary artery bypass surgery. Circulation 60:250–259
Ivert T, Landou C (1981) Changes in coronary artery disease five years after coronary bypass surgery. Scand J Thorac Cardiovasc Surg 15:187–198
Robert EW, Guthaner DF, Wexler L et al (1978) Six-year clinical and angiographic follow-up of patients with previously documented complete revascularization. Circulation 58:1194–1199
Mintz GS, Painter JA, Pichard AD et al (1995) Atherosclerosis in angiographically “normal” coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol 25:1479–1485
Tobis JM, Mallery J, Mahon D et al (1991) Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation 83:913–926
Mintz GS, Nissen SE, Anderson WD et al (2001) American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 37:1478–1492
Korshunov VA, Schwartz SM, Berk BC (2007) Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol 27:1722–1728
Bakker EN, Pistea A, Spaan JA et al (2006) Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res 99:86–92
Pistea A, Bakker EN, Spaan JA et al (2005) Flow inhibits inward remodeling in cannulated porcine small coronary arteries. Am J Physiol Heart Circ Physiol 289:H2632–H2640
Ward MR, Tsao PS, Agrotis A et al (2001) Low blood flow after angioplasty augments mechanisms of restenosis: inward vessel remodeling, cell migration, and activity of genes regulating migration. Arterioscler Thromb Vasc Biol 21:208–213
Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodeling. N Engl J Med 330:1431–1438
Toggweiler S, Urbanek N, Schoenenberger AW et al (2010) Analysis of coronary bifurcations by intravascular ultrasound and virtual histology. Atherosclerosis 212:524–527
Mintz GS, Pichard AD, Popma JJ et al (1997) Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol 29:268–274
Mintz GS, Kent KM, Pichard AD et al (1997) Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation 95:1791–1798
Fujii K, Carlier SG, Mintz GS et al (2005) Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol 96:1476–1483
Higashikuni Y, Tanabe K, Yamamoto H et al (2007) Relationship between coronary artery remodeling and plaque composition in culprit lesions: an intravascular ultrasound radiofrequency analysis. Circ J 71:654–660
Mintz GS, Popma JJ, Pichard AD et al (1995) Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1,155 lesions. Circulation 91:1959–1965
Mintz GS, Pichard AD, Kent KM et al (1998) Interrelation of coronary angiographic reference lumen size and intravascular ultrasound target lesion calcium. Am J Cardiol 81:387–391
Sangiorgi G, Rumberger JA, Severson A et al (1998) Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 31:126–133
Conflict of interest
Dr. Shang receives fund from Zhejiang Provincial Nature Science Foundation (Y2090393) and grant from Boston Scientific Corporation, China. Drs. Pu and Guo receive grants from Boston Scientific Corporation, China. Drs. Mintz and Maehara receive grants/research support from Boston Scientific Corporation and Volcano Corporation. Drs. Leon and Stone are the members of the advisory boards for Boston Scientific Corporation. Dr. Moses is a consultant for Boston Scientific Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shang, Y., Mintz, G.S., Pu, J. et al. Bypass to the left coronary artery system may accelerate left main coronary artery negative remodeling and calcification. Clin Res Cardiol 102, 831–835 (2013). https://doi.org/10.1007/s00392-013-0598-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-013-0598-6