Abstract
Objective
The ECG plays a central role in the rapid diagnosis of acute myocardial infarctions (MI). In haemodynamically instable patients, adhesion of electrodes sometimes is difficult and assessing ECGs through layers of clothes has not been done so far. A novel capacitive measurement of ECG signals is possible without skin contact. Whether this technical innovation can be used in patients with MI is unclear.
Methods
We evaluated a capacitive ECG system (cECG) in patients with anterior and inferior ST elevation MI (STEMI) as compared to patients without ST elevations in anterior and inferior leads. The cECG was assessed using a sensor array consisting of 15 electrodes of which the classical leads I, II, III, aVL, aVF and V1–V3 were calculated from. 66 patients were included in the study. In addition to the conventional ECG (kECG) the novel cECG was registered before reperfusion therapy was started.
Results
In a first round, 19 patients presented with anterior MI, 23 with inferior MI, and 7 either with left bundle branch block or lateral MI. Regarding anterior MI, a significant correlation (P < 0.05) was found between ST elevations in leads I, aVL, V2 and V3 comparing cECG and kECG. In inferior MI, there was only a significant correlation (P < 0.05) in lead III between cECG and kECG, but not in II and aVF. Therefore, 17 additional patients were included in the study by placing an additional electrode further away from the sensor array on the chest. ST elevations now correlated in all inferior leads II, III and aVF (P < 0.05) as measured in 9 patients with inferior MI. In addition, in 8 patients an inferior MI was correctly ruled out.
Conclusion
It is possible to identify STEMIs by cECG. This innovative technique could play an important role in the pre-hospital period as well as in the hospital.
Similar content being viewed by others
Introduction
ECG
The ECG is a central diagnostic tool for heart diseases since it was described by Einthoven more than one century ago [1]. Besides the assessment and differentiation of arrhythmias, the ECG becomes more and more important in the early diagnosis of myocardial infarction in addition to rapid treatment as urged by current guidelines of many cardiological societies [2, 3]. This is even more important in the pre-hospital period to quickly assess ST elevation myocardial infarction (STEMI), transfer ECG signals to a PCI centre, and to initiate adequate and rapid reperfusion therapy [4].
Unfortunately, when trying to assess an ECG in haemodynamically instable patients such as patients being in pre-shock or shock, the adhesion of conventional electrodes to the skin sometimes is difficult if not impossible due to the wet skin caused by higher perspiration. Moreover, in certain situations it is difficult or simply too time-consuming to undress the patient before acquiring an ECG. Therefore, novel ECG techniques are required.
Capacitive electrodes
Capacitive electrodes are a dry, non-contact alternative to conventional wet bioelectric electrodes, preventing the need of an electrolyte gel and, in contrast to common dry electrodes, enabling the measurement through insulating materials like hair or even clothes. Such capacitive electrode principles are well known since the end of the 1960s. In the field of cardiac diagnostics, one main research field was focused on classic diagnostic systems for ECG measurement in clinical environments [5–7]. New fields of application were also investigated due to the insulating behavior of these electrodes [8]. Integration in chairs or beds and wearable devices shows motivating results [9–11]. The main problem of capacitive electrodes is the sensitivity to movement artifacts. Movements of the skin relative to the sensor cause in great artifact amplitudes. Thus, some groups also focused on the reduction of these artifacts for practical application of this technology [12].
The main concept of capacitive measurements of bioelectric signals is the capacitive coupling between the skin and a metallic face inside the sensor. This electrode is placed close to the skin with an insulating material between the electrode face and the skin. An ultra-high input impedance amplifier is connected to the metallic face. This guarantees a low input corner frequency below 100 mHz for the capacitively coupled biosignal. The coupling process is based on the electrical displacement currents caused by the changing potential distribution of the heart. These displacement currents are mirrored on the face of the electrode and amplified by this special amplifier. To prevent disturbances from outside electrical fields, shielding (normally active and passive) is needed for a stable behavior of the electrode. Due to the amplification and active shielding, capacitive electrodes are active. As already shown in prior studies on healthy volunteers, the capacitive (cECG) gives exactly the same information as the conventional (kECG) when electrodes are placed at the same positions and comparable signals when electrodes are placed in the configuration of the sensor array used in this study [13, 14].
Whether this technical innovation could be used for detection of ST elevations in patients with myocardial infarction was unclear. Therefore, we evaluated a novel cECG system in patients with STEMI.
Methods
System
The used cECG system is based on a sensor array with 15 capacitive electrodes (Fig. 1a, b), which is well described in prior studies [13, 14]. Capacitive electrodes can be placed on the patient without any preparation, so it does not make a difference between placing one electrode or even multiple electrodes. The array configuration enables the combination of standard time-based leads and 2D spatial measurements (body surface potential mapping, BSPM). Moreover, this configuration allows a cable free electrode placement and a portable and compact system. Nevertheless, it does not realize a replacement for a standard 12-channel ECG, because the 12-channel configuration always need cabling and single electrode fixation on the standard lead positions.
a View of the back of the tablet PC with the sensor array consisting of 15 electrodes. b A single capacitive electrode compared to the size of a 2 € coin. c Positioning of the tablet PC/cECG device on the chest of a patient. d Relative position of the electrodes on the chest of the patient. O3 is a fixed point in the fourth intercostal space on the right (being V1 in the conventional ECG). Electrode O5 serves as capacitive reference for differential measurement. The leads after Einthoven and Goldberger (I, II, III, aVR, aVL, aVF) are shown through the blue triangle (and the blue box on the right) whereas the Wilson leads (V1–V3) are represented by the red electrodes (and the red box on the right)
In this study which was performed between March 2009 and May 2010, the focus was on the detection of ST elevations with this array configuration and not on BSPM, therefore standard lead approximations were extracted out of the sensor array data to better compare the measured ECG with a conventional ECG. A positive vote by the local ethics committee of the University Medicine Göttingen was requested before the study and granted.
In our study, 49 patients were initially included who presented in our Heart Centre of the University Hospital Göttingen (Table 1), either in our Chest Pain Unit (CPU) certified by the German Cardiac Society or were transported directly to our catheter lab through an emergency physician. Of course, all patients were asked to agree to participate in the study. This happened prior to the cECG measurements in the catheter lab by signature of the patients. cECGs were assessed before reperfusion therapy was started and compared to a conventional ECG (kECG). The capacitive ECGs were measured by placing the system on the patient’s undressed chest (as shown graphically in Fig. 1d) with a galvanic grounding electrode connected to one arm of the patient. This grounding electrode decreases the influence of external disturbing fields. Each cECG measurement lasted up to 30 s and was performed in parallel to the preparation in the catheter lab shortly before starting the acute coronary angiography. There were no predefined exclusion criteria. Patients were included in an all comer fashion. Main inclusion criteria were the existence of both a capacitive and a conventional ECG. Because we did not want to delay reperfusion therapy in STEMI patients we did not assess cECGs in those instances where it may have taken too long (patient was already on the cath table, ready to start the catheterization).
Extraction
The system was placed on the undressed chest (Fig. 1c) to get extracted Einthoven/Goldberger leads and three Wilson leads (V1–V3). Due to the different shapes of the body, the best electrodes for the extraction were selected subsequently. Only if one of these electrodes was not available, electrodes close to the predefined electrodes (Fig. 1d: blue triangle and red marked electrodes) were selected. For the extraction procedure, the leads were calculated by the following equations (Fig. 1d):
This extraction is based on signals measured in the near field of the heart, so this extraction cannot generate the same leads measured on the standard positions. One question of this study apart from the principled sensitivity to capacitive electrodes for ST elevations was the reliability of this extraction approach in the clinical setup. The main purpose of the lead extraction is not to get real standard leads since this is limited by the array configuration. The extraction, however, may help the medical staff to quickly assess the ECG in its known way.
After an interim evaluation (49 patients), an external capacitive electrode was added to the setup due to the low detection rate of inferior myocardial infarctions. This external electrode was placed on the left side of the patient’s lower back as a foot electrode to enlarge the triangle of Einthoven (Fig. 6a). 17 additional patients were successfully measured with this external electrode not only to better assess but also to rule out confidently an inferior MI.
Statistics
Both ECGs were measured manually due to the parameters Q, R, S voltage, ST segment height and T voltage. These measurements were directly correlated between kECG and cECG for all patients. For statistical evaluation, simple linear regressions were used. A P value of 0.05 was used as a significance level. Standard deviation was used throughout the manuscript.
Results
ECGs of acute anterior STEMI
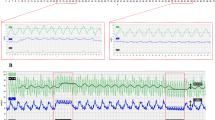
Figure 2 shows a typical original conventional (kECG; Fig. 2a) as well as a capacitive ECG (cECG; Fig. 2b) recording from a patient with an acute anterior myocardial infarction. In the kECG one can clearly see the ST elevations in leads I, aVL, V1–V3. In accordance with this, ST elevations can be assessed with the help of the cECG having similar ST elevations in leads I, aVL, V2–V3. Coronary angiogram in this patient revealed a complete blockade of the proximal LAD which was instantaneously dilated and stented successfully. Moreover, this patient had a non-significant 40% blockage of the right coronary artery. The corresponding electrode array and original recordings are presented in Fig. 2c with electrodes O1 and O11 showing no clear signals due to the patient′s anatomy (skinny chest). This array displays the array electrodes referenced to a Wilson central terminal calculated out of the selected R, L and F channels. They represent Wilson leads with a higher spatial resolution around V1 and V3. Nevertheless, as shown in Fig. 2b, adequate surface ECG could be convincingly assessed by the cECG system.
All data for 49 patients (Table 2) were analyzed for leads I, II, III, aVL, aVF, and V1–V3 independent of the localization of the MI. Significant correlations were found for ST elevations in leads I, III, aVL, and V2–V3 pointing to the fact that especially anterior leads seem to correlate well.
This is underlined when only analyzing the patients with anterior MI with respect to leads I, aVL, and V1–V3 (Table 3). With respect to their ST elevations, R amplitudes, as well as T wave amplitudes, all data correlated significantly between kECG and cECG, except for lead V1. As an example, values for lead V2 are shown graphically in Fig. 3 in addition.
ECGs of acute inferior STEMI
Figure 4 shows an original conventional (Fig. 4a) as well as a capacitive ECG (Fig. 4b) from a patient with an inferior myocardial infarction. In the kECG one can clearly see the ST elevations in leads II, III, aVF. In accordance with this, ST elevations can be assessed, although not as clearly as in the kECG, with the help of the cECG showing also ST elevations in leads II, III, aVF. The coronary angiogram in this patient revealed a complete blockade of the proximal RCA (with masses of fresh thrombi) which was dilated and stented successfully. Moreover, this patient had a 60% blockage of the LAD and a 50% blockage of the RD1. The corresponding electrode array and original recordings are presented in Fig. 4c with the electrode O1 showing no clear signals due to the patient’s anatomy. Nevertheless, as shown in Fig. 4b adequate surface ECG could be assessed.
When analyzing all data from 23 patients with inferior MI (Table 4) for leads III, aVF with respect to their ST elevations, only lead III correlated significantly between kECG and cECG (Fig. 5). This is underlined by the fact that the other classical inferior leads II and aVF even do not correlate well when analyzing all 49 patients (Table 2).
ECGs of inferior STEMI with additional electrode
To improve the representation of inferior infarctions an external capacitive electrode was added in the second part of the trial. The electrode was placed at the patient’s lower back as a foot electrode to enlarge the triangle for lead extraction (Fig. 6a, green triangle). Altogether, 17 patients were measured this way of which 9 patients presented with inferior infarctions. Original examples are presented in Fig. 6b with significant correlations now for ST elevation and T wave amplitudes of lead aVF as an example being graphically displayed in Fig 6c. The new results showed even significant correlations (P < 0.05) of ST elevations in leads II, III and aVF (Table 5) when only including the 9 patients with inferior infarction. In summary, cECG shows the ability for a correct and fast STEMI diagnosis. Due to the limitations out of the array size, an additional electrode was used to enlarge the diagnostic information in the case of inferior infarction.
Time parameters
In addition to the ECG amplitudes, time parameters were assessed. As an example for the ECGs in the patients with anterior MI, QT interval in lead V2 was 408 ± 48 ms for cECG as compared to 410 ± 46 ms for kECG on average. The individual values (n = 19) correlated significantly (P = 0.006; r 2 = 0.364).
In addition, ECGs in the patients with inferior MI (n = 23) had mean QT intervals in lead III with 426 ± 58 ms for cECG as compared to 424 ± 40 ms for kECG. The individual values correlated significantly (P = 0.023; r 2 = 0.224) even without additional electrode. This was expected due to the fact that the capacitive sensor measures the same electrical signal source as galvanic electrodes.
Discussion
In the present proof of concept study, we present data in patients with acute ST myocardial infarction showing that the portable cECG system based on an electrode array is a promising approach for the fast STEMI diagnosis, shown on patients with anterior MI and with the help of an additional electrode at the lower back also on patients with inferior MI.
Due to the current guidelines for the treatment of myocardial infarctions, rapid ECG assessment is mandatory in patients with chest pain and acute coronary syndromes contributing to a reduction in door- and contact-to-balloon times to primary angioplasty [2–4, 15–17]. Usually, conventional ECG assessment including undressing takes about 2–3 min. Not only time is critical but also being able to adequately measure ECGs, e.g. during difficult clinical situations when the patient is in shock or when a patient is resuscitated. Regular electrodes in these situations may not be easily fixed to the patient’s chest and limbs and therefore alternative techniques are warranted. Quick assessment of an ECG within a few seconds (about 15–30 s) as suggested by the current cECG even through the clothes may be of great clinical advantage.
In the current study, we investigated 66 patients with MI and found clear correlations in the ECG alterations analyzed including ST elevations, T wave amplitudes, as well as R amplitudes between kECG and cECG. This was true from the beginning of the study for patients with anterior myocardial infarctions for the classical leads I, aVL, and V2–V3. The anterior wall is well mapped by Wilson’s leads because it is in close distance to the left anterior chest. Therefore, locally placed capacitive electrodes can adequately measure ECG signals as shown by the results of the current study.
This, however, is different in the situation where the inferior wall of the heart is damaged through an acute MI. Here, usually leads II, III, and aVF which are measured by electrodes placed at the left and right arm versus left leg are needed. In acute situations, one can also place electrodes at the left and right shoulder versus left lower chest or back. Our results show that without an additional electrode at the left lower back the capacitive electrodes on the chest are not sufficient to adequately assess inferior MI because only a significant correlation for ST elevation was found in lead III. One possible explanation is that ST elevations were the highest in lead III. This was consistent in the conventional kECG as well as in the capacitive cECG resulting in a better correlation as compared to II and aVF (although there was at least a trend in aVF with P = 0.1598). Further assessment in 9 patients with inferior MI show that with the simple help of this additional lower back electrode even the capacitive electrodes on the upper left chest are able to adequately assess ST elevation in all three leads II, III, and aVF. The array configuration and, therefore, the lead extraction limits the correlation values in comparison to the 12 channel ECG, so further studies are necessary to improve the lead approximation (especially for lead V1) and also to extend the sensor range to V4–V6. This limitation (also with respect to additional leads V7–V9) can be easily approached by extension of the current pilot system by a larger electrode array which covers additional regions for the missing Wilson leads and by detaching the capacitive electrodes from the tablet PC to give more flexibility. Such a system is already under development.
Because of the study design and the changes of the measurement configuration (additional electrode) during the study, a reliable and needed analysis of sensitivity and specificity should be addressed in a different study design. Furthermore, the outcome of this study will be used to improve the shown system and realize these further studies. Therefore, a new and larger non-inferiority trial (FIDET) for patients presenting with acute coronary syndrome (ACS) was initiated to investigate sensitivity and specificity of the novel cECG.
Some other limitations of this study have to be discussed. Due to the design of the study, the time for measuring the cECG was strictly limited by the start of the reperfusion therapy. Because of the limited body adaption provided by the presented system and the different shapes of the patients’ chests, an optimal adaption of all electrodes was not possible in all cases (see electrodes O1 and O11 in Fig. 2c, as well as electrode O1 in Fig. 4c). Nevertheless, the adaption was controlled for the relevant electrodes (Fig. 1d) before starting the measurement without a reduction in the quality of the cECG signals.
Movement artifacts may in principal have complicated our results. Because of the adaption mechanism of the electrodes and the patients’ supine position, movement artifacts were effectively reduced during the measurements.
The absolute amplitudes of the capacitive ECG are different from kECG, resulting from the different recording positions. Previous evaluation showed the same amplitude, when both types of electrodes were placed at the same positions. In our study, we used exemplarily a cutoff value of 40 μV in lead aVF in the cECG looking at the n = 9 patients with inferior STEMI (as diagnosed by the kECG) and we could correctly assess 8 out of 9 patients in the cECG. In contrast to the amplitudes, the QT intervals correlated well between cECG and kECG.
Finally, another limitation of the current study is that only 9 patients with inferior STEMI were tested after changing the configuration which is not enough to proof the clinical applicability yet but points to the fact that further clinical testing is needed.
In summary, we could show for the first time using capacitive electrodes that this technique is able to assess myocardial infarctions in a clinical environment. Further work will focus on the reliability and on the system design. We believe that this innovative technique may help in the near future to assess ECGs more easily even in clinically difficult situations. Moreover, these electrodes may be also used in the future as an integrated part to a mattress in the tables in the catheter lab or in patients′ beds without additional electrode fixation on the chest.
References
Einthoven W (1895) Ueber die Form des menschlichen Electrocardiogramms. Pflüger, Archiv für die Gesammte Physiologie des Menschen und der Thiere. 60:101–123
Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, ESC Committee for Practice Guidelines (CPG), Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Silber S, Aguirre FV, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Di Mario C, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GY, Rutten F (2008) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the european society of cardiology. Eur Heart J 29:2909–2945
Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC Jr, Writing Committee Members, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW (2004) 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines: developed in collaboration with the canadian cardiovascular society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation 117:296–329
Diercks DB, Kontos MC, Chen AY, Pollack CV Jr, Wiviott SD, Rumsfeld JS, Magid DJ, Gibler WB, Cannon CP, Peterson ED, Roe MT (2009) Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. J Am Coll Cardiol 53:161–166
Richardson PC (1967) The insulated electrode: a pasteless electrocardiographic technique. In: 20th Annual conference on engineering in medicine and biology, vol 9
Searle A, Kirkup L (2000) A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol Meas 21:271–283
Prance RJ, Debray A, Clark TD, Prance H, Nock M, Harland CJ, Clippingdale AJ (2000) An ultra-low-noise electrical-potential probe for human-body scanning. Meas Sci Technol 11:291–297
Clippingdale AJ, Prance RJ, Clark TD, Watkins C (1994) Ultrahigh impedance capacitively coupled heart imaging array. Rev Sci Instrum 65:269–270
Lim YG, Kim KK, Park KS (2006) ECG measurement on a chair without conductive contact. IEEE Trans Biomed Eng 53:956–959
Lim YG, Kim KK, Park KS (2007) ECG recording on a bed during sleep without direct skin‐contact. IEEE Trans Biomed Eng 54:718–725
Steffen M, Aleksandrowicz A, Leonhardt S (2007) Mobile noncontact monitoring of heart and lung activity. IEEE Trans Biomed Circuits Syst 1:250–257
Ueno A, Akabane Y, Kato T, Hoshino H, Kataoka S, Ishiyama Y (2007) Capacitive sensing of electrocardiographic potential through cloth from the dorsal surface of the body in a supine position: a preliminary study. IEEE Trans Biomed Eng 54:759–766
Oehler M, Ling V, Melhorn K, Schilling M (2008) A multichannel portable ECG system with capacitive sensors. Physiol Meas 29:783–793
Oehler M, Schilling M, Esperer HD (2009) Capacitive ECG system with direct access to standard leads and body surface potential mapping. Biomed Technik/Biomed Eng 54:329–335
Müller UM, Eitel I, Eckrich K, Erbs S, Linke A, Möbius-Winkler S, Mende M, Schuler GC, Thiele H (2011) Impact of minimising door-to-balloon times in ST-elevation myocardial infarction to less than 30 min on outcome: an analysis over an 8-year period in a tertiary care centre. Clin Res Cardiol 100:297–309
Birkemeyer R, Rillig A, Koch A, Miljak T, Kunze M, Meyerfeldt U, Steffen W, Soballa M, Ranke C, Prassler R, Benzing A, Jung W (2010) Primary angioplasty for any patient with ST-elevation myocardial infarction? Guideline-adherent feasibility and impact on mortality in a rural infarction network. Clin Res Cardiol 99:833–840
Maier LS, Schirmer SH, Walenta K, Jacobshagen C, Böhm M (2009) Hotline update of clinical trials and registries presented at the German Cardiac Society Meeting 2009. Clin Res Cardiol 98:413–419
Acknowledgments
Dr. Maier is funded by the Deutsche Forschungsgemeinschaft (DFG) through the Clinical Research group KFO155 (MA 1982/2-2) and a Heisenberg grant (MA 1982/4-1).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. B. Weil and M. Oehler contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Weil, M.B., Oehler, M., Schilling, M. et al. First clinical evaluation of a novel capacitive ECG system in patients with acute myocardial infarction. Clin Res Cardiol 101, 165–174 (2012). https://doi.org/10.1007/s00392-011-0377-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-011-0377-1