Abstract
Background
We investigated the safety, feasibility and usefulness for closure of PFO with the new nitinol meshwire PFO-occluder device (Occlutech Figulla®-single layer occluder) with an unique braiding technology which allows a 50% reduction of meshwork material on the left atrial side in combination with a greater flexibility as compared to the Amplatzer® occluder device.
Methods
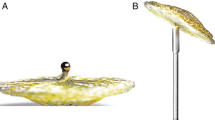
The retention discs of the new PFO Occlutech Figulla® single layer device (23/25 mm) are connected by a 3 mm waist in the centre with only one right atrial side hub. The left atrial disc is a single flat layer covered by an ultrathin polyethylene terephthalate (PET) patch. We investigated the safety, feasibility and usefulness for closure of PFO in a multicenter clinical trial. Indications for closure included cryptogenic stroke with evidence of a patent foramen ovale in transesophageal echocardiography (PFO max. diameter 13 mm according to sizing balloon). The device was implanted in 36 patients (mean age 57, 18–80 years) by means of fluoroscopy and transesophageal echocardiography (TEE) using a 9 French delivery sheath and employing a femoral vein approach. Both acetylsalicylacid 100 mg/d (6 months) and clopidogrel 75 mg/d (3 months) were administered post interventional. A transthoracal (TTE) and transesophageal echocardiography follow-up examination was performed after 1, 2 and 6 months (TTE day 30 and 180; TEE day 60).
Results
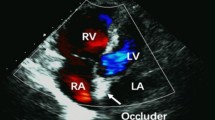
The device was successfully implanted in 36 pts. In one patient PFO implantations was attempted but not crossed with a guide wire. Perioperativly there were no major in-hospital-adverse events or complications thromboembolism, occluder dislodgement, infection or myocardial infarction. One patient had transient atrial fibrillation 2 h after implantation, which terminated medically after 12 h. TEE studies in the remaining 35 pts (one pt was unwilling to further participate) showed a residual shunt in 8.6% (3/35) after 60 days and a left-to-right shunt in 2.6% (1/35) of pts. After 180 days one pt with severe arteriosclerotic heart disease and A.carotic stenosis revealed a stroke without evidence of cardioembolic origin or devices thrombosis.
Conclusions
The novel Occlutech Figulla® PFO N single layer device appears to be safe, feasible and useful for PFO closure despite a 50% reduction of the meshwire, no distal hub and an improved flexibility of the left atrial disc.



Similar content being viewed by others
References
Azarbal B, Tobis J, Suh W, Chan V, Dao C, Gaster R (2005) Association of interatrial shunts and migraine headaches: Impact of transcatheter closure. J Am Coll Cardiol 45:489–492
Beitzke A, Schuchlenz H, Beitzke M et al (2002) Interventioneller Verschluß von Foramen ovale und Vorhofseptumdefekten nach paradox embolischen Ereignissen. Z Kardiol 91:693–700
Braun MU, Ehrhard K, Strasser RH, Haass M (2002) Katheterinterventioneller Verschluss eines ventiloffenen Foramen ovale über eine zusätzliche transseptale Punktion. Z Kardiol 91(8):659–662
Braun M, Gliech V, Boscheri A, Schoen S, Gahn G, Reichmann H, Haass M, Schraeder R, Strasser RH (2004) Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism: periprocedural safety and mid-term follow-up results of three different device occluder systems. Eur Heart J 25:424–430
Fischer D, Fuchs M, Schaefer A et al (2008) Transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Procedural and follow-up results after implantation of the Starflex® occluder device with conjunctive intensified anticoagulation regimen. J Interv Cardiol 21(2):183–189
Hagen P, Scholz D, Edwards W (1984) Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984, Jan. i 59:17–20
Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A (2007) Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007 Nov 29 357:2262–2268
Konstantinides S, Just H (2000) Ventiloffenes Formane ovale: Konservative oder operative Therapie. Z Kardiol 89(2):63–71
Lock JE (2000) Patent foramen ovale is indicted, but the case hasn’t gone to trial. Circulation 101:838
Martín F, Sánchez PL, Doherty E et al (2002) Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation 106:1121–1126
Mas JL, Arquizan C, Lamy C et al (2001) Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 345:1740–1746
Mas JL, Zuber M (1995) Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French study group on patent foramen ovale and atrial septal aneurysm. Am Heart J 130:1083–1088
Morandi E, Anzola GP, Angeli S, Melzi G, Onorato E (2003) Transcatheter closure of patent foramen ovale: a new migraine treatment? J Interv Cardiol 16:39–42
Mügge A, Daniel WG, Angermann C et al (1995) Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation 91:2785–2792
Reisman M, Christofferson RD, Jesurum J, Olsen JV, Spencer MP, Krabill KA, Diehl L, Aurora S, Gray WA (2005) Migraine headache relief after transcatheter closure of patent foramen ovale. J Am Coll Cardiol 45:493–495
Satler LF (2002) Should PFO closure devices be used “Off Lable”. Catheter Cardiovasc Interv 56:527
Sievert H, Taaffe M (2005) Patent foramen ovale: the jury is still out. Eur Heart J 25:361–362
Stone DA, Godard J, Corretti MC et al (1996) Patent foramen ovale: association between the degree of shunt by contrast transesophageal echocardiography and the risk of future ischemic neurologic events. Am Heart J 131:158–161
Windecker S, Wahl A, Chatterjee T et al (2000) Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation 101:893–898
Acknowledgment
The trial was supported by a grant provided by Occlutech Jena®.
Conflict of interest statement Dr. Florian Krizanic and Prof. Dr. H.R. Figulla are consultants of Occlutech ®. Prof. Dr. H.R. Figulla has shares in Occlutech ® Jena. The other authors reported no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krizanic, F., Sievert, H., Pfeiffer, D. et al. Clinical evaluation of a novel occluder device (Occlutech®) for percutaneous transcatheter closure of patent foramen ovale (PFO). Clin Res Cardiol 97, 872–877 (2008). https://doi.org/10.1007/s00392-008-0699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-008-0699-9