Abstract
Purpose
Colorectal cancer (CRC) is increasingly diagnosed in individuals aged < 50 years, resulting in advocacy of screening from age 45 years. Despite existing knowledge associating CRC with conventional adenomas, the significance of sessile serrated lesions (SSLs) on the burden of CRC is less detailed. We aimed to provide contemporary estimates for SSL prevalence and examine patient and procedure factors associated with SSL detection.
Methods
Retrospective observational study examining associations between SSL and conventional adenoma detection, polyp histopathology, patient, and procedure characteristics in an outpatient colonoscopy unit over 12 months.
Results
From 2097 colonoscopies, SSL detection was 13.8% overall and 12.5% in patients < 50 years. SSLs were mostly proximal in location (64%), and SSL detection was significantly higher in females compared with males (16.2% vs. 11.7%, p = 0.003), particularly in those < 50 years (16.8% vs. 8.6%, p < 0.001). In multivariable analysis, SSL detection was associated with female sex (adjusted odds ratio [aOR] 1.48, 95% confidence interval [CI] 1.15–1.91), synchronous conventional adenoma detection (aOR 1.36, 95% CI 1.04–1.78) and BMI ≥ 25 kg/m2 (aOR 1.34, 95% CI 1.02–1.77). Conventional adenoma detection was 33.6% and associated with age ≥ 50 years (aOR 3.57, 95% CI 2.84–4.47) and synchronous SSL detection (aOR 1.36, 95% CI 1.03–1.79).
Conclusions
We observed age and sex disparities in polyp types and prevalence in this outpatient colonoscopy population. SSLs were most prevalent in females aged < 50 years, suggesting a potential increased susceptibility of young females to SSLs and CRC. Our findings may have implications for the design of CRC screening programs.
Similar content being viewed by others
Introduction
CRC is the third most common cancer worldwide and a second-leading cause of cancer deaths. The burden of CRC is particularly high in Australia, ranking among the top five regions for CRC incidence [1]. Risk factors include polyps, diverticular disease, and a low-fibre diet [2]. As CRC rates continue to rise globally, ongoing efforts are being made to better understand the changing epidemiology, risk factors, prevention, screening, and treatment of CRC. Rising CRC rates in people < 50 years of age is increasingly being observed [3].
Approximately two-thirds of CRCs are derived from conventional adenomas, henceforth referred to as adenomas. However, the serrated neoplasia pathway is another important contributor to CRC, accounting for approximately one-third of sporadic CRCs [4,5,6]. The World Health Organisation (WHO) currently identifies three different types of serrated polyps: hyperplastic polyps (HP), sessile serrated lesions (SSL−previously known as sessile serrated adenomas/polyps), and traditional serrated adenomas (TSA) [7]. SSLs are of contemporary interest, as their detection may be challenging and awareness regarding their prevalence and malignant potential is increasing [4, 5]. Furthermore, SSLs seem to be associated with a disproportionately high number of interval CRCs compared to their reported prevalence [8]. This has raised questions about whether current screening paradigms for CRC appropriately target SSL detection.
Histopathologically, the WHO recently defined SSLs as “serrated polyps with ≥ 1 unambiguous distorted crypt”, which is more sensitive than the previous diagnostic criteria from 2010 requiring “pathological features in two or three adjacent crypts” [7, 9]. This has led to a considerable proportion of previously diagnosed HPs being reclassified as SSLs [5, 10]. Molecularly, the serrated pathway is known to exhibit high amounts of cytosine-phosphate-guanine (CpG) island methylation and can express microsatellite instability. This is also known as the CpG island methylator phenotype (CIMP) [11, 12]. The reported prevalence of SSLs varies from 1.1 to 15% depending on the study population and year examined, which may reflect the use of different diagnostic criteria and geographical variations. The variation in SSL detection is also observed among studies in the same geographic region and the similar time period and may reflect advancing knowledge, individual endoscopist and pathologist experience, and improvements in colonoscope technology.
There is also ongoing controversy regarding risk factors associated with SSLs, with high variability seen for both endoscopist SSL detection rate and histopathological identification [4, 13,14,15,16,17]. Factors contributing to these inconsistencies include (a) the flat morphology of SSLs and their frequent location in the proximal colon, (b) periodic updates of SSL histopathological diagnostic criteria by the WHO, most recently in 2019, and (c) the fact that SSLs rarely bleed, resulting in low detection utilising the faecal immunochemical test (FIT) as a sole screening tool [4, 15, 18]. In Australia, the current minimum SSL detection rate of 4%, set as a quality measure by the Gastroenterological Society of Australia (GESA) for colonoscopy recertification of endoscopists [19], appears somewhat arbitrary and is probably inadequate relative to emerging knowledge.
Aims
We aimed to provide contemporary estimates for SSL prevalence and identify patient and procedure characteristics associated with SSL detection.
Methods
Study design
Our study was a retrospective observational study of an outpatient colonoscopy population at a non-tertiary hospital. Patient and procedure characteristics were recorded from the clinical records. Study approval was obtained from the East Metropolitan Health Service Governance, Evidence, Knowledge, Outcomes Committee (activity approval 32886) and did not require written or informed consent because of the low-risk, retrospective, and non-interventional design of the study.
Study population and colonoscopy characteristics
All colonoscopies performed in an outpatient day surgery unit between Jan 1 and Dec 31, 2019, were included in the study (n = 2097). Patient data were obtained from referral letters and clinical records. Data recorded included age, sex, body mass index (BMI), and colonoscopy indication. Patients undergoing surveillance for inflammatory bowel disease (IBD) were not excluded. Patient characteristics that were not consistently documented, such as ethnicity, smoking status, and alcohol consumption, were not included. Colonoscopy data obtained included endoscopist speciality, quality of bowel preparation, withdrawal time, colonoscopy findings including the size and location of colorectal polyps, the presence of mass lesions, and incidental findings such as diverticular disease and haemorrhoids.
Colorectal polyp histopathology data
Polyp histopathology reports were reviewed, and histologic characteristics including the presence of dysplasia were recorded. Polyp types of interest were SSLs, adenomas, HPs, and TSAs. Adenomas were subclassified as tubular, tubulovillous, or villous adenomas. Advanced adenomas were defined as adenomas with a size ≥ 10 mm, a prominent villous component, or high-grade dysplasia. Clinically significant serrated polyps (CSSPs) were defined as SSLs, TSAs, proximal colon HPs ≥ 5 mm, or HPs ≥ 10 mm anywhere in the colon [20]. Molecular pathology information was not available in histopathology reports. We defined proximal colorectal polyps as polyps in the caecum, ascending colon, hepatic flexure, and transverse colon, while distal colorectal polyps were at or distal to the splenic flexure (i.e. splenic flexure, descending, sigmoid colon, and rectum) as previously described [21]. Individual endoscopist adenoma detection rate (ADR) and SSL detection rates (SDRs) were calculated.
Statistical analysis
Data are summarised as median (interquartile range), means (standard deviation), or proportions. Univariate analyses were performed to examine associations between the presence of SSLs or adenomas and individual patient and procedure characteristics using Pearson Chi-square tests or Student’s t tests. Missing data were omitted from analyses. All p values were reported as two-sided and were interpreted at the 5% level of significance. For correlations between SSL detection and adenoma detection, we included data on endoscopists who performed more than 100 colonoscopies during the study period. Multivariable logistic regression analyses were conducted, including patient demographic and colonoscopy-related characteristics that were significantly associated with the polyp types in univariate analyses. HPs were not included in multivariable predictive models for SSLs and adenomas, as they were not considered clinically significant. The outcome variables are SSL, CSSP, and adenoma detection. Odds ratios (OR) and 95% confidence intervals (CI) for SSL, CSSP, and adenoma detection are reported. OR is presented as adjusted OR (aOR) in multivariable analysis. All statistical analyses were performed with the Statistical Packages for the Social Sciences (SPSS), version 26 (IBM Corp., Armonk, NY, USA).
Results
Patient cohort factors
Amongst the 2097 colonoscopies performed in the study, 1365 (65%) involved polypectomy. The median patient age was 54 (41–63) years, and 46.6% of patients were female. The mean BMI was 26.5 (5.5) kg/m2. The mean age did not differ significantly, comparing males versus females (51.8 years for males, 52.0 years for females, p = 0.798), nor did mean BMI (26.4 kg/m2 for males, 26.6 kg/m2 for females, p = 0.406). Reasons for colonoscopy are summarised in Table 1. Some colonoscopies were performed for multiple indications.
Procedure factors
Colonoscopies were performed by specialist gastroenterologists (64.9%) or general surgeons (35.1%), with nine colonoscopists performing more than 100 colonoscopies each during the study year. Colonoscopes used were the Olympus 180 series. Bowel preparation was good (reported as excellent or adequate) in 93% of colonoscopies based on colonoscopist impression. A total of 29.8% of patients had diverticular disease. Chromoendoscopy use was documented in < 1% of colonoscopies. Withdrawal times were only reported in 50% of colonoscopies, however, were consistently longer than 6 min where documented.
Polyp detection
Fifteen pathologists were involved in polyp diagnosis in our study. SSL detection was 13.8% overall, with most SSLs located in the proximal colon (64.4% proximal vs. 35.6% distal, p < 0.001) and having no dysplasia (99%). Approximately 30.4% of SSLs without dysplasia were > 10 mm. The percentage of SSLs located in the proximal colon was not significantly different between sexes or between patients under and over 50 years (p > 0.05). Only one patient had a formal diagnosis of serrated polyposis, as consistent with recent WHO guidelines for gastrointestinal neoplasia [7]. The distribution of polyps is shown in Table 2. Most adenomas were tubular adenomas with low-grade dysplasia (90.3%), followed by tubulovillous adenomas with low-grade dysplasia (8.1%). Six patients had adenocarcinoma (0.29% of all colonoscopies). Nine out of 2097 patients had incomplete polyp data.
Univariate analysis
Associations between patient cohort factors and SSL detection
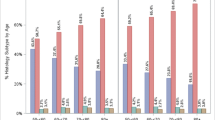
The associations between patient cohort factors and SSL detection are summarised in Table 3. SSL detection was not significantly different between patients < 50 and ≥ 50 (12.5% and 14.7%, respectively, p = 0.17). However, comparing males and females, SSL detection was higher in females than males (16.2% vs. 11.7% respectively, p = 0.003). This sex difference was most significant in patients aged under 50 years (females 16.8% vs. males 8.6%, p < 0.001). By contrast, there was no significant sex difference in SSL detection in patients aged above 50 years (15.8% vs. 13.7%, respectively, p = 0.67). The distribution of SSL detection by sex and age categories is shown in Fig. 1. Amongst younger patients aged < 40 years, SSL detection was significantly higher in females compared to males (18.4% vs. 8.5%, respectively, p = 0.002). Considering other patient characteristics, SSL detection was higher in patients with BMI ≥ 25.0 kg/m2 compared to BMI < 25.0 kg/m2 (15.2% vs. 11.3%, respectively, p = 0.02). A total of 17.8% of colonoscopies had a positive FIT status and of these, 18.0% included detection of an SSL. SSL detection was not predicted by colonoscopy indication nor a positive FIT (p = 0.10).
Associations between patient cohort factors and adenoma detection
Adenoma detection was significantly higher in patients aged ≥ 50 compared with < 50 years (45.0% vs. 16.8%, respectively, p < 0.001). This finding was consistent in males (45.7% vs. 15.6%, p < 0.001) and females (44.2% vs. 18.0%, p < 0.001). There was no significant difference in adenoma detection between sexes (males 33.9% vs. females 33.2%, p = 0.30). Adenoma detection was highest in patients with screening/surveillance as the only indication for colonoscopy (38.5%) and was significantly associated with a positive FIT (OR 1.96, 95% CI 1.40–2.73) and BMI ≥ 25.0 kg/m2 (OR 1.56, 95% CI 1.29–1.90).
Associations between procedure characteristics and SSL detection rates (SDR)
Table 4 summarises the associations between procedure characteristics and SSL detection. SDRs ranged between 9 and 16.8% among colonoscopists. SSL detection was higher in patients with synchronous adenomas and HPs. Furthermore, SDR was significantly correlated with individual endoscopist ADR (r = 0.83, n = 9, p = 0.002). The relationship between individual SDR and ADR is shown in Fig. 2. There was no association between SSL detection and the presence of diverticular disease.
Associations between procedure characteristics and adenoma detection
Adenoma detection ranged between 21.5 and 45% among colonoscopists. Coincident colonoscopy findings associated with adenoma detection were synchronous SSL detection (OR 1.45. 95% CI 1.12–1.87) and the presence of diverticular disease (OR 1.78, 95% CI 1.47–2.16).
Multivariable analysis
Patient cohort and procedure factors associated with SSL detection and CSSP detection
Factors included in multivariable logistic regression analysis for SSL detection were patient age (categorical < versus ≥ 50 years), sex, BMI (categorical < versus ≥ 25 kg/m2), synchronous adenoma detection, and bowel preparation. SSL detection was associated with female sex (aOR 1.48, 95% CI 1.15–1.91), synchronous adenoma detection (aOR 1.36, 95% CI 1.04–1.78), and BMI ≥ 25 kg/m2 (aOR 1.34, 95% CI 1.02–1.77). With HPs included in the model, factors associated with SSL detection were female sex (aOR 1.48, 95% CI 1.15–1.91), synchronous adenoma detection (aOR 1.37, 95% CI 1.04–1.79), and synchronous HP detection (aOR 1.64, 95% CI 1.26–2.15). For CSSP detection, multivariable logistic regression included SSL detection, BMI, synchronous adenoma detection, and bowel preparation. Factors associated with CSSP detection were female sex (aOR 1.40, 95% CI 1.11–1.75) and synchronous adenoma detection (aOR 1.43, 95% CI 1.12–1.83).
Patient cohort and procedure factors associated with adenoma detection
Factors included in multivariable logistic regression analysis for adenoma detection were patient age (categorical < versus ≥ 50 years), BMI (categorical < versus ≥ 25 kg/m2), a positive FIT, synchronous SSL detection, diverticular disease, and bowel preparation. Adenoma detection was associated with age ≥ 50 (aOR 2.24, 95% CI 1.66–3.00) and synchronous SSL detection (aOR 1.79, 95% CI 1.30–2.48).
Discussion
In this outpatient colonoscopy population that included adults aged between 18 and 75 years and various indications, we found a SDR of 13.8% overall and 12.5% in patients < 50 years. The rate of SSL detection was significantly higher in females compared to males, with this sex difference being most significant in patients < 50 years of age. Female sex, synchronous adenoma detection, and BMI > 25 kg/m2 significantly increased the odds of SSL detection in multivariable analysis. None of the three indication categories (blood loss, non-bleeding gastrointestinal symptoms, or surveillance/screening) were associated with SSL detection. The majority (64%) of SSLs were found in the proximal colon. There was no significant association between SSL detection and a diagnosis of diverticular disease.
Risk factors for SSL and adenoma detection
Age and sex
Our findings suggest females under 50 years may be at increased risk for SSL detection. This was independent of the quality of bowel preparation, which would be expected to influence the yield of polyp detection. CRC screening guidelines in Australia are currently not sex-specific, with two-yearly FIT recommended for all screening average-risk individuals aged 50–74 years, and colonoscopy performed following a positive result [22]. The association between sex and SSL detection, however, is unclear. Some studies identify equal prevalence among males and females, whereas others find either males or females to have a higher prevalence. This variability in sex association is observed regardless of geographical region. Australian studies have reported either no association with sex or association with female sex [16, 23]. On the other hand, European studies report either no association or association with the male sex [24, 25]. Studies derived from the US populations generally report no association with sex, whereas South American studies have reported an association with female sex [14, 26,27,28].
There are several potential reasons why our study, and others, exhibit considerable variability in SSL sex associations and SSL prevalence. Firstly, the dates of publication of the above studies range from 2008 to 2019, suggesting that different histopathological criteria have been used to identify SSLs [14, 16, 23,24,25,26,27,28]. The most recent SSL histopathological criteria are more sensitive than those used previously, possibly contributing to our relatively high-observed prevalence. Moreover, there is high variability in both endoscopist and histopathologist detection/diagnosis of SSLs, with recorded endoscopist detection rates of 0.6–20.1% and pathologist classification rates of 0.5–12.0% [4, 17, 29]. In addition to changing pathological criteria, it is likely the above variation is due to differences in endoscopist/histopathologist skills since both colonoscopy and histopathological analysis are subjective techniques. The histopathological assessment of all polyps in our study was provided by specialist histopathologists from the state specialist tertiary pathology service. Finally, different studies may have involved patient cohorts with inherently different characteristics and susceptibilities to SSL risk due to unknown confounding variables. The reliability and generalisability of previous research are therefore unclear. Contemporary, large-scale studies are needed to provide more robust evidence regarding risk factors for SSL detection.
Existing data is also inconsistent regarding age as a risk factor for SSLs, with some studies reporting advanced age as a risk factor and other studies finding no correlation. Reasons for this variability are similar to those regarding sex. A recent Australian study by Kim et al. [3] identified rising CRC rates in patients aged < 50 years, particularly in males. Furthermore, another recent paper by Sehgal et al. [30] concluded that colonoscopy at ages 45–49 was associated with a considerable decrease in CRC incidence in both males and females. Lash et al. [27] reported that dysplasia and carcinoma progression were disproportionately higher in female patients with SSLs compared to male patients. In view of the above findings, and the emergence of new diagnostic criteria for SSLs, further data is needed to determine the adequacy of current screening guidelines and benchmarks for SSL detection.
In contrast to SSLs, we found that adenoma detection was significantly higher in patients ≥ 50 than < 50 years, with no significant difference between males and females. We also noted that while SSLs and CSSPs were more prevalent in females up to age 59 years, no sex difference was seen with adenomas. While our findings concur with previous studies in terms of age associations, previous literature has shown higher adenoma detection in males compared to females [31,32,33]. With regard to baseline characteristics between our cohort’s males and females, mean BMI and age did not significantly differ. There may be other confounding risk factors creating a bias that was not captured in our data and contributed to a relatively high prevalence of adenomas in females. These include smoking, ethnicity, other past medical histories, particular incidental findings, family history of cancer, and diet.
Other risk factors
Other risk factors for SSL detection exhibit varied significance in the literature, with tobacco, alcohol, and the white race being among the most consistently identified [4, 18]. Many of these risk factors could not be examined in our study given their inconsistent reporting. BMI was associated with SSL detection in univariate and multivariable analysis excluding HPs. The significance of such findings, however, remains largely unclear, as while studies show associations between serrated lesion detection or adenoma detection and BMI [34, 35], few studies focus specifically on SSLs. Whether or not sex hormones and adiposity contribute to SSL risk in young women remains unclear. Such a link between sex hormones and early colorectal carcinogenesis has been suggested in a previous study by Hang et al.; however, specific associations for SSLs were not explored [36]. Although the diverticular disease was not associated with SSL detection in our study, there was an association between diverticular disease and adenomas in our univariate analysis and also in previous studies and meta-analyses [2, 37, 38]. Further studies using contemporary diagnostic criteria for SSLs may elucidate associations between SSLs and the risk factors above. Finally, synchronous adenomas were associated with SSL detection in both univariate and multivariate analyses. This is consistent with previous studies suggesting SSLs increase the risk of synchronous and metachronous neoplasia [39, 40].
Colonoscopy indication
Although we found that no colonoscopy indication was associated with SSL detection, this is a complex variable to study. Patients often had numerous indications, making the association of individual indications with SSL detection difficult to analyse. Furthermore, because indications were grouped into three different categories, conclusions can only be made about indication categories as a whole. We also found synchronous adenoma detection was associated with SSL detection. Therefore, if a particular indication was associated with adenoma detection, this would likely associate with SSL detection regardless of whether it was an independent covariate. Significantly, FIT status was not associated with SSL detection, consistent with the fact that SSLs rarely bleed [4, 16]. This means that while some studies support earlier initiation of FIT for increasing rates of CRC in average-risk patients < 50 years, such an approach may provide inadequate protection for females < 50 years who may be more susceptible to SSLs [3]. Whether there are specific characteristics that would justify screening with colonoscopy in females < 50 years remains to be explored.
SSL prevalence and current detection benchmarks
The standard quality indicator for colonoscopist performance in screening colonoscopy is the ADR, i.e., the proportion of colonoscopies performed in which at least one adenoma is detected and removed [41, 42]. The currently accepted benchmark ADR for endoscopists is ≥ 25% [43], which has been used by some studies to derive benchmarks for SSL detection. The correlation between ADR and SDR, however, remains uncertain, with certain studies showing moderate to high correlation (as reflected in our findings) and others suggesting otherwise [18, 44, 45]. The Cancer Council Australia (CCA) recommends a serrated lesion detection rate of > 10% [46]. Nevertheless, this benchmark includes HPs, which are more prevalent than SSLs but are not considered premalignant [4]. As for SSL-specific benchmarks, GESA proposes a 4% detection threshold for SSLs [19]. Such a rate, however, may be inadequate considering the rising prevalence rates of SSLs and our detection rate of 13.8%. Our findings, therefore, support the revision of benchmarks for SSL detection as additional data emerges. Previous studies indicate that the mortality benefit of colonoscopy screening has been limited to distal CRC [47,48,49]. This suggests that enhancing the detection of SSLs, which are mostly proximal in location [4], will significantly improve the effectiveness of colonoscopy screening.
Significance of SSLs in patients under 50 years
The significance of our finding of higher rates of SSLs in younger females is uncertain, particularly given it is a novel finding that is not described extensively in the literature. A study from the USA provides reassurance that proximal SSLs have a relatively low likelihood of progressing to cancer in young people [50]. This is attributed to the fact that most young-onset CRC (onset < 50 years) is distal and exhibits different molecular characteristics than SSLs [51]. A more recent study by Hamoudah et al., however, suggests an increased risk of metachronous advanced colorectal neoplasia when small, serrated lesions and adenomas coexist [52]. This means that when accounting for other factors like synchronous adenoma detection and variable SSL prevalence and sex distribution, the association between SSLs and young-onset CRC becomes less clear. Our study found that SSLs frequently coexist with adenomas−a major precursor to young-onset CRC [50]. Furthermore, when considering Australian data, we find that 24% of cases of young-onset CRC diagnosed between 2001 and 2008 were proximal in location, meaning some could have been contributed to by SSLs or risk factors shared with adenomas [53]. While some studies have demonstrated a high prevalence of SSLs in a general colonoscopy population [54], few studies have investigated the significance of a potentially higher prevalence of SSLs in patients under 50 years. The fact that 16.8% of females under 50 years in our study had SSLs may warrant further consideration and examination of how such detection rates may correlate with young-onset CRC. Although a study by Lash et al. [27] demonstrates slow rates of SSL progression to dysplasia and carcinoma, another study by Oono et al. suggests that such progression may also occur rapidly [55]. Therefore, while most SSLs in our study occurred without dysplasia, the natural history of SSLs and the risk they pose to females under 50 years with potential susceptibility to SSLs remains unclear. Finally, although young-onset CRC tends to occur distally whereas SSLs tend to occur proximally, a considerable proportion of SSLs in our study were distal (> 30%), including in patients < 50 years. Further studies using prospective, multicentre, and contemporary histopathological data are required to elucidate the significance of these findings.
Strengths and limitations
Our study has several strengths, including a large and reasonably well-characterised population and reliance on histopathologic descriptions of polyps. We observed a high photo-documented caecal intubation rate and reasonably high-quality bowel preparation. Additionally, our data involved specialist colonoscopists without outlier SDRs, which adds generalisability to our findings and reduces the influence of inter-endoscopist variability. We also provide data that is likely to reflect contemporary diagnostic criteria for serrated lesions. As for limitations, the retrospective design of our study means it is limited by some missing or inconsistently reported data (for example smoking and ethnicity) and unknown biases. It is also unclear what proportion of patients had a prior colonoscopy. However, less than 20% of colonoscopies were performed for surveillance (the majority, approximately 15%, being for previous polyp detection), suggesting that most procedures were not for polyp surveillance.
Data regarding IBD in our study was limited as IBD patients comprised < 1% of our patient cohort. Nevertheless, associations between IBD and SSLs remain unclear [56]. In terms of colonoscopy characteristics, withdrawal times were not consistently documented, which leads to uncertainty regarding adherence to the recommended > 6-min withdrawal times [57]. Similarly, chromoendoscopy use was poorly documented, suggesting the technique was rarely used in this cohort. Furthermore, bowel preparation was recorded based on colonoscopist impression (graded as excellent, adequate, fair, inadequate, or poor) rather than validated scoring systems such as the Boston Bowel Prep Score. [58] Regarding histopathology, there is likely to be variability in our study in terms of diagnostic criteria for SSLs given that multiple pathologists (15) were involved in polyp diagnosis. It was, therefore, difficult to ascertain what proportion of pathologists utilised the 5th edition versus the 4th edition of the WHO criteria for SSL diagnosis. However, all pathologists worked in a tertiary teaching hospital capacity. As newer criteria are more sensitive for SSL diagnosis, our findings likely underestimate rather than overestimate the true prevalence of SSLs. Addressing the above limitations requires further, high-quality, large prospective studies.
Conclusion
Our study identified an SSL prevalence of 13.8% in an outpatient colonoscopy population, with females < 50 years being at considerable risk. Other associated factors included the presence of synchronous adenomas, which indicates a potential subsequent risk of advanced colorectal neoplasia. These findings suggest that current SDR benchmarks and CRC screening guidelines may be inadequate, particularly if females < 50 years are at increased risk of CRC via the serrated-neoplasia pathway. Whether or not differences in sex-hormone levels with age play a role in the increased susceptibility of young females to SSLs remains to be elucidated. Further studies are required to resolve the significance of our findings.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Burkitt DP (1971) Epidemiology of cancer of the colon and rectum. Cancer 28(1):3–13. https://doi.org/10.1002/1097-0142(197107)28:1%3c3::AID-CNCR2820280104%3e3.0.CO;2-N
Kim J, Dobson B, Ng Liet Hing C, Cooper M, Lu CT, Nolan G et al (2020) Increasing rate of colorectal cancer in younger patients: a review of colonoscopy findings in patients under 50 at a tertiary institution. ANZ J Surg 90(12):2484–2489. https://doi.org/10.1111/ans.16060
Crockett SD, Nagtegaal ID (2019) Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology 157(4):949–966. https://doi.org/10.1053/j.gastro.2019.06.041
Jaravaza DR, Rigby JM (2020) Hyperplastic polyp or sessile serrated lesion? The contribution of serial sections to reclassification. Diagn Pathol 15(1):1–9. https://doi.org/10.1186/s13000-020-01057-0
Snover DC (2011) Update on the serrated pathway to colorectal carcinoma. Hum Pathol 42(1):1–10
WHO Classification of Tumours Editorial Board (2019) Digestive system tumours: Who Classification of Tumours: 1, 5th edn. World Health Organization, Philippines, p 635
IJspeert JE, Vermeulen L, Meijer GA, Dekker E (2015) Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 12(7):401. https://doi.org/10.1038/nrgastro.2015.73
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, 4th edn. World Health Organization, Switzerland, p 417
Schramm C, Kaiser M, Drebber U, Gruenewald I, Franklin J, Kuetting F et al (2016) Factors associated with reclassification of hyperplastic polyps after pathological reassessment from screening and surveillance colonoscopies. Int J Colorectal Dis 31(2):319–325. https://doi.org/10.1007/s00384-015-2404-6
Jass J (2007) Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50(1):113–130. https://doi.org/10.1111/j.1365-2559.2006.02549.x
Jang HS, Shin WJ, Lee JE, Do JT (2017) CpG and non-CpG methylation in epigenetic gene regulation and brain function. Genes 8(6):148. https://doi.org/10.3390/genes8060148
Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L et al (2014) Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol 38(2):158–166
Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S et al (2010) Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 105(12):2656–2664
Limketkai BN, Lam-Himlin D, Arnold MA, Arnold CA (2013) The cutting edge of serrated polyps: a practical guide to approaching and managing serrated colon polyps. Gastrointest Endosc 77(3):360–375. https://doi.org/10.1016/j.gie.2012.11.013
Ismail A, Lall V, Ayonrinde OT (2021) Should the high prevalence of sessile serrated lesions in patients aged below 50 years influence screening colonoscopy recommendations? J Gastroenterol Hepatol 36(7):2022–2023. https://doi.org/10.1111/jgh.15511
Gourevitch RA, Rose S, Crockett SD, Morris M, Carrell DS, Greer JB et al (2018) Variation in pathologist classification of colorectal adenomas and serrated polyps. Am J Gastroenterol 113(3):431
O’Connell BM, Crockett SD (2017) The clinical impact of serrated colorectal polyps. Clin Epidemiol 9:113. https://doi.org/10.2147/CLEP.S106257
Gastroenterological Society of Australia. Colonoscopy Recertification Program [Internet]. [place: unknown]: Gastroenterological Society of Australia [cited 2021 May 17]. Available from: https://recert.gesa.org.au/viewCriteria.php.
Park SK, Park SH, Yang HJ, Jung YS, Park JH, Sohn CI et al (2020) Simple proxies for detection of clinically significant serrated polyps and data for their benchmarks. J Gastroenterol Hepatol 35(8):1365–1371. https://doi.org/10.1111/jgh.14977
Jackson CS, Vega KJ (2015) Higher prevalence of proximal colon polyps and villous histology in African-Americans undergoing colonoscopy at a single equal access center. J Gastrointest Oncol 6(6):638
Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer [Internet]. Sydney (NSW): Cancer Council Australia; [cited 2021 May 17]. Available from: https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer.
Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL (2009) Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol 62(6):516–518. https://doi.org/10.1136/jcp.2008.061960
Schramm C, Janhsen K, Hofer J-H, Toermer H, Stelzer A, Stenschke F et al (2018) Detection of clinically relevant serrated polyps during screening colonoscopy: results from seven cooperating centers within the German colorectal screening program. Endoscopy 50(10):993–1000
Álvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J et al (2013) Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc 78(2):333–341. https://doi.org/10.1016/j.gie.2013.03.003
Sanaka MR, Gohel T, Podugu A, Kiran RP, Thota PN, Lopez R et al (2014) Adenoma and sessile serrated polyp detection rates: variation by patient sex and colonic segment but not specialty of the endoscopist. Dis Colon Rectum 57(9):1113–1119
Lash RH, Genta RM, Schuler CM (2010) Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 63(8):681–686. https://doi.org/10.1136/jcp.2010.075507
Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL et al (2019) Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 156(1):254–272. https://doi.org/10.1053/j.gastro.2018.08.063
Farris AB, Misdraji J, Srivastava A, Muzikansky A, Deshpande V, Lauwers GY et al (2008) Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol 32(1):30–35
Sehgal M, Ladabaum U, Mithal A, Singh H, Desai M, Singh G (2021) Colorectal cancer incidence after colonoscopy at ages 45–49 or 50–54 years. Gastroenterology 160(6):2018–2028. https://doi.org/10.1053/j.gastro.2021.02.015
Waldmann E, Heinze G, Ferlitsch A, GessI I, Sallinger D, Jeschek P et al (2016) Risk factors cannot explain the higher prevalence rates of precancerous colorectal lesions in men. Br J Cancer 115(11):1421–1429. https://doi.org/10.1038/bjc.2016.324
Brenner H, Altenhofen L, Stock C, Hoffmeister M (2014) Incidence of colorectal adenomas: birth cohort analysis among 4.3 million participants of screening colonoscopy. Cancer Epidemiol Biomark Prev 23(9):1920–1927. https://doi.org/10.1158/1055-9965.EPI-14-0367
Vatn MH, Stalsberg H (1982) The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer 49(4):819–825. https://doi.org/10.1002/1097-0142(19820215)49:4<819::AID-CNCR2820490435>3.0.CO;2-D
Bailie L, Loughrey MB, Coleman HG (2017) Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology 152(1):92–104. https://doi.org/10.1053/j.gastro.2016.09.003
Neugu AI, Lee WC, Garbowski GC, Waye JD, Forde KA, Treat MR et al (1991) Obesity and colorectal adenomatous polyps. J Natl Cancer Inst 83(5):359–361. https://doi.org/10.1093/jnci/83.5.359
Hang D, He X, Kværner AS et al (2021) Plasma sex hormones and risk of conventional and serrated precursors of colorectal cancer in postmenopausal women. BMC Med 19:18. https://doi.org/10.1186/s12916-020-01895-1
Jaruvongvanich V, Sanguankeo A, Wijarnpreecha K, Upala S (2017) Risk of colorectal adenomas, advanced adenomas and cancer in patients with colonic diverticular disease: systematic review and meta-analysis. Dig Endosc 29(1):73–82. https://doi.org/10.1111/den.12701
Singh S, Singh PP, Murad MH, Singh H, Samadder JN (2014) Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 109(9):1375–1389
Gao Q, Tsoi KK, Hirai HW, Wong MC, Chan FK, Wu JC et al (2015) Serrated polyps and the risk of synchronous colorectal advanced neoplasia: a systematic review and meta-analysis. Am J Gastroenterol 110(4):501–509
Schreiner MA, Weiss DG, Lieberman DA (2010) Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 139(5):1497–1502. https://doi.org/10.1053/j.gastro.2010.06.074
Pu LZCT, Singh G, Rana K, Nakamura M, Yamamura T, Krishnamurthi S et al (2020) Polyp detection rate as a surrogate for adenoma and sessile serrated adenoma/polyp detection rates. Gastrointest Tumors 7(3):74–82. https://doi.org/10.1159/000505622
Rembacken B, Hassan C, Riemann J, Chilton A, Rutter M, Dumonceau J-M et al (2012) Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 44(10):957–968
Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB et al (2015) Quality indicators for colonoscopy. Gastrointest Endosc 81(1):31–53. https://doi.org/10.1016/j.gie.2014.07.058
Anderson JC, Butterly LF, Weiss JE, Robinson CM (2017) Providing data for serrated polyp detection rate benchmarks: an analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc 85(6):1188–1194. https://doi.org/10.1016/j.gie.2017.01.020
Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK (2011) Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 9(1):42–46. https://doi.org/10.1016/j.cgh.2010.09.013
Appleyard M, Brown G, Raftopoulos S, Sutherland T, Singh R, Butt J et al. Quality of colonoscopy [Internet]. [updated 2019 March 25; cited 2021 May 18]. Available from: https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer/Colonoscopy_surveillance/Quality_of_colonoscopy.
Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L (2009) Association of colonoscopy and death from colorectal cancer. Ann Intern Med 150(1):1–8. https://doi.org/10.7326/0003-4819-150-1-200901060-00306
Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M (2011) Protection from colorectal cancer after colonoscopy: a population-based, case–control study. Ann Intern Med 154(1):22–30. https://doi.org/10.7326/0003-4819-154-1-201101040-00004
Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN (2010) The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 139(4):1128–1137. https://doi.org/10.1053/j.gastro.2010.06.052
Segev L, Kalady MF, Plesec T et al (2020) The location of premalignant colorectal polyps under age 50: a further rationale for screening sigmoidoscopy. Int J Colorectal Dis 35:529–535. https://doi.org/10.1007/s00384-020-03504-2
Bettington M, Brown I, Rosty C, Walker N, Liu C, Croese J et al (2019) Sessile serrated adenomas in young patients may have limited risk of malignant progression. J Clin Gastroenterol 53(3):e113–e116
Hamoudah T, Vemulapalli KC, Alsayid M, Van J, Ma K, Jakate S et al (2022) Risk of total metachronous advanced neoplasia in patients with both small tubular adenomas and serrated polyps. Gastrointest Endosc. https://doi.org/10.1016/j.gie.2022.02.015 (Epub ahead of print)
Boyce S, Nassar N, Lee CY, Suen MK, Al Zahrani S, Gladman MA (2016) Young‐onset colorectal cancer in New South Wales: a population‐based study. Med J Aust 205(10):465–70.53. https://doi.org/10.5694/mja16.00237
Bettington M, Walker N, Rahman T, Vandeleur A, Whitehall V, Leggett B et al (2017) High prevalence of sessile serrated adenomas in contemporary outpatient colonoscopy practice. Intern Med J 47(3):318–323. https://doi.org/10.1111/imj.13329
Oono Y, Fu K, Nakamura H, Iriguchi Y, Yamamura A, Tomino Y et al (2009) Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci 54(4):906–909. https://doi.org/10.1007/s10620-008-0407-7
Bossard C, Denis MG, Bézieau S, Bach-Ngohou K, Bourreille A, Laboisse CL et al (2007) Involvement of the serrated neoplasia pathway in inflammatory bowel disease-related colorectal oncogenesis. Oncol Rep 18(5):1093–1097
Rembacken B, Hassan C, Riemann JF, Chilton A, Rutter M, Dumonceau JM et al (2012) Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 44(10):957–968
Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC (2009) The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 69(3):620–625. https://doi.org/10.1016/j.gie.2008.05.057
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Vidit Lall and Ali Galalah Mostafa Ismail (equal first authors) contributed equally to the following: data acquisition, data analysis, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Oyekoya T Ayonrinde (senior author) was responsible for the study’s conception and design, interpretation of data, and critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lall, V., Ismail, A.G.M. & Ayonrinde, O.T. Disparate age and sex distribution of sessile serrated lesions and conventional adenomas in an outpatient colonoscopy population–implications for colorectal cancer screening?. Int J Colorectal Dis 37, 1569–1579 (2022). https://doi.org/10.1007/s00384-022-04191-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04191-x