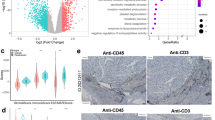
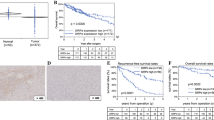
Abstract
Purpose
This study investigated the expression of interleukin 32 (IL-32) in hepatoblastoma, the most common primary pediatric liver tumor, and its possible roles in tumorigenesis.
Methods
IL-32 expression was investigated in two hepatoblastoma cell lines (Hep G2 and HuH 6) in the steady state and after co-culture with macrophages by RNA-seq analysis and RT-qPCR, and after stimulation with chemotherapy. Cultured macrophages were stimulated by IL-32 isoforms followed by RT-qPCR and western blot analysis. IL-32 immunohistochemical staining (IHC) was performed using specimens from 21 hepatoblastoma patients. Clustering analysis was also performed using scRNA-seq data downloaded from Gene Expression Omnibus.
Results
The IL-32 gene is expressed by hepatoblastoma cell lines; expression is upregulated by paracrine cell–cell communication with macrophages, also by carboplatin and etoposide. IL-32 causes protumor activation of macrophages with upregulation of PD-L1, IDO-1, IL-6, and IL-10. In the patient pool, IHC was positive only in 48% of cases. However, in the downloaded dataset, IL-32 gene expression was negative.
Conclusion
IL-32 was detected in hepatoblastoma cell lines, but not in all hepatoblastoma patients. We hypothesized that stimulation such as chemotherapy might induce expression of IL-32, which might be a critical mediator of chemoresistance in hepatoblastoma through inducing protumor activation in macrophages.






Similar content being viewed by others
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- Bcl-2:
-
B-cell lymphoma 2
- CD163:
-
Cluster of differentiation 163
- EGF:
-
Endothelial growth factor
- HB:
-
Hepatoblastoma
- HMDMs:
-
Human monocyte derived macrophages
- Iba1:
-
Ionized calcium-binding adapter molecule 1
- IC50:
-
Half maximal inhibitory concentration
- IDO-1/2:
-
Indoleamine dioxygenase-1/2
- IHC:
-
Immunohistochemical staining
- IL-1β/6/10/32/34:
-
Interleukin-1 beta/6/10/32/34
- Kyn pathway:
-
Kynurenine pathway
- LPS:
-
Lipopolysaccharide
- M-CSFR:
-
Macrophage colony stimulating factor receptor
- MMP-2,7,9:
-
Matrix metalloproteinases-2,7,9
- MTB:
-
Mycobacterium tuberculosis
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NKs:
-
Natural killer cells
- PCNSL:
-
Primary central nervous system lymphoma
- PDGF:
-
Platelet-derived growth factor
- PD-L1/2:
-
Programmed death ligand 1/2
- PLAA:
-
Phospholipase A2-activating protein
- RNA-seq:
-
Ribonucleic acid sequencing
- RT-qPCR:
-
Real time quantitative polymerase chain reaction
- SCC:
-
Squamous cell carcinoma
- STAT3:
-
Signal transducer and activator of transcription 3
- TAMs:
-
Tumor-associated macrophages
- Tet-On/Off:
-
Tetracycline-On/Off
- TGF-β:
-
Tumor growth factor beta
- TME:
-
Tumor microenvironment
- TNF-α:
-
Tumor necrosis factor alpha
- VEGF:
-
Vascular endothelial growth factor
References
Spector LG, Birch J (2012) The epidemiology of hepatoblastoma. Pediatr Blood Cancer 59:776–779
López-Terrada D, Alaggio R, De Dávila MT, et al (2014) Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Modern Pathology 27:472–491
Cairo S, Armengol C, De Reyniès A et al (2008) Hepatic Stem-like Phenotype and Interplay of Wnt/β-Catenin and Myc Signaling in Aggressive Childhood Liver Cancer. Cancer Cell. https://doi.org/10.1016/j.ccr.2008.11.002
Dehne N, Mora J, Namgaladze D et al (2017) Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol 35:12–19
Yin M, Li X, Tan S et al (2016) Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Investig. https://doi.org/10.1172/JCI87252
Egawa M, Mukai K, Yoshikawa S et al (2013) Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4. Immunity. https://doi.org/10.1016/j.immuni.2012.11.014
Yang Q, Guo N, Zhou Y et al (2020) The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B 10:2156–2170
Irie T, Yoshii D, Komohara Y et al (2022) IL-34 in hepatoblastoma cells potentially promote tumor progression via autocrine and paracrine mechanisms. Cancer Med. https://doi.org/10.1002/cam4.4537
Li L, Irie T, Yoshii D et al (2022) M-CSFR expression in the embryonal component of hepatoblastoma and cell-to-cell interaction between macrophages and hepatoblastoma. Med Mol Morphol. https://doi.org/10.1007/s00795-022-00323-y
Sloot YJE, Smit JW, Joosten LAB, Netea-Maier RT (2018) Insights into the role of IL-32 in cancer. Semin Immunol 38:24–32
Zaidan SM, Leyre L, Bunet R et al (1988) (2019) Upregulation of IL-32 Isoforms in Virologically Suppressed HIV-Infected Individuals: Potential Role in Persistent Inflammation and Transcription from Stable HIV-1 Reservoirs. J Acquir Immune Defic Syndr. https://doi.org/10.1097/QAI.0000000000002185
Netea MG, Azam T, Lewis EC et al (2006) Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-γ-dependent mechanism. PLoS Med. https://doi.org/10.1371/journal.pmed.0030277
Shim S, Lee S, Hisham Y et al (2022) A Paradoxical Effect of Interleukin-32 Isoforms on Cancer. Front Immunol 13:422
Komohara Y, Kawauchi R, Makiyama E et al (2017) Selective depletion of cultured macrophages by magnetite nanoparticles modified with gelatin. Exp Ther Med. https://doi.org/10.3892/etm.2017.4640
Komohara Y, Hasita H, Ohnishi K et al (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. https://doi.org/10.1111/j.1349-7006.2011.01945.x
Komohara Y, Niino D, Saito Y et al (2013) Clinical significance of CD163+ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. https://doi.org/10.1111/cas.12167
Fujiwara Y, Komohara Y, Ikeda T, Takeya M (2011) Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. https://doi.org/10.1111/j.1349-7006.2010.01772.x
Song H, Bucher S, Rosenberg K et al (2022) Single-cell analysis of hepatoblastoma identifies tumor signatures that predict chemotherapy susceptibility using patient-specific tumor spheroids. Nat Commun 13:4878. https://doi.org/10.1038/s41467-022-32473-z
Aass KR, Kastnes MH, Standal T (2021) Molecular interactions and functions of IL-32. J Leukoc Biol. https://doi.org/10.1002/JLB.3MR0620-550R
Kang YH, Park M-Y, Yoon D-Y et al (2012) Dysregulation of overexpressed IL-32α in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κB and Bcl-2. Cancer Lett. https://doi.org/10.1016/j.canlet.2011.12.023
Sorrentino C, Di Carlo E (2009) Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.200903-0400OC
Zeng Q, Li S, Zhou Y et al (2014) Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. https://doi.org/10.1016/j.cyto.2013.09.017
Zhang F, Suarez G, Sha J et al (2009) Phospholipase A2-activating protein (PLAA) enhances cisplatin-induced apoptosis in HeLa cells. Cell Signal. https://doi.org/10.1016/j.cellsig.2009.02.018
Goda C, Kanaji T, Kanaji S et al (2006) Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. https://doi.org/10.1093/intimm/dxh339
Tsai CY, Wang CS, Tsai MM et al (2014) Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-13-1221
Yan H, Dong M, Liu X et al (2019) Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. https://doi.org/10.1016/j.canlet.2019.01.012
Kim SW, Roh J, Park CS (2016) Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. https://doi.org/10.4132/jptm.2016.08.08
Maggi LB, Weber JD (2013) Forget Transcription: Translation Is Where the Action Is. Mol Cell Biol. https://doi.org/10.1128/mcb.00231-13
Acknowledgements
We thank Mr. Takenobu Nakagawa and Mrs. Yuka Watanabe for their technical assistance.
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 20H03459 to Y.K., and No. 21K08622 to D.Y). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design: AA, YK and TH; data collection: AA, LL, HH, TI, DY, HY, YF, SE, MH and SS; data analysis and interpretation: AA and YK; writing the manuscript (original draft preparation, review & editing): AA and YK; critical revision: TH.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflicts of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent to be included in the study was obtained from the patients’ caregivers. The study design and proposal were approved by the Institutional Review Board (IRB) at Kumamoto University (IRB No. 2224).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
383_2023_5557_MOESM1_ESM.tif
Supplementary file1 Image showing results of western blot analysis. Chemiluminescence (left) and chemiluminescence with molecular weight markers (right) for PD-L1 (a) and IDO-1 (b) are shown (TIF 310 KB)
383_2023_5557_MOESM2_ESM.tif
Supplementary file2 IC50 for carboplatin and etoposide. HepG2 (a) and HuH-6 (b) cell lines were cultured with various concentrations of carboplatin and etoposide for 48 hours and then the cell viability assay was performed (TIF 106 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adawy, A., Li, L., Hirao, H. et al. Potential involvement of IL-32 in cell-to-cell communication between macrophages and hepatoblastoma. Pediatr Surg Int 39, 275 (2023). https://doi.org/10.1007/s00383-023-05557-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05557-0