Abstract
Purpose
Immunological abnormalities have been hypothesized as a pathogenesis of biliary atresia (BA). We previously investigated the frequency and function of circulating regulatory T-cells (Tregs) and reported no differences compared to controls. However, the local Treg profile remains uncertain. We aimed to investigate the frequency of Tregs in BA liver tissues.
Methods
The number of lymphocytes, CD4+ cells, and CD4+FOXP3+ Tregs infiltrating the portal tract and the percentage of Tregs among CD4+ cells of BA and control patients were visually counted. The correlation between these data and clinical indicators was also examined.
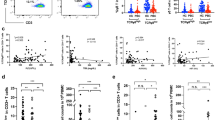
Results
The number of lymphocytes, CD4+ cells, and CD4+FOXP3+ Tregs was higher in the BA group. However, the percentage of Tregs among CD4+ cells was similar in both groups. Each parameter was correlated with serum γ-GTP, but there was no clear association with liver fibrosis, jaundice clearance, and native liver survival.
Conclusion
The number of Tregs infiltrating the portal tract was higher in BA patients. However, the infiltration of lymphocytes was also generally increased. Tregs appear to be unsuccessful in suppressing progressive inflammation in BA patients, despite recruitment to local sites. Investigation of Treg function in the local environment is warranted.


Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bezerra JA, Wells RG, Mack CL et al (2018) Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology 68:1163–1173. https://doi.org/10.1002/hep.29905
Chardot C (2006) Biliary atresia. Orphanet J Rare Dis 1:28. https://doi.org/10.1186/1750-1172-1-28
Asai A, Miethke A, Bezerra JA (2015) Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 12:342–352. https://doi.org/10.1038/nrgastro.2015.74
Nizery L, Chardot C, Sissaoui S et al (2016) Biliary atresia: clinical advances and perspectives. Clin Res Hepatol Gastroenterol 40:281–287. https://doi.org/10.1016/j.clinre.2015.11.010
Nio M (2017) Japanese biliary atresia registry. Pediatr Surg Int 33:1319–1325. https://doi.org/10.1007/s00383-017-4160-x
Mack CL, Feldman AG, Sokol RJ (2012) Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 32:307–316. https://doi.org/10.1055/s-0032-1329899
Sokol RJ, Mack C, Narkewicz MR, Karrer FM (2003) Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr 37:4–21. https://doi.org/10.1097/00005176-200307000-00003
Hartley JL, Davenport M, Kelly DA (2009) Biliary atresia. Lancet 374:1704–1713. https://doi.org/10.1016/S0140-6736(09)60946-6
Wang J, Xu Y, Chen Z et al (2020) Liver immune profiling reveals pathogenesis and therapeutics for biliary atresia. Cell 183:1867-1883.e26. https://doi.org/10.1016/j.cell.2020.10.048
Lakshminarayanan B, Davenport M (2016) Biliary atresia: a comprehensive review. J Autoimmun 73:1–9. https://doi.org/10.1016/j.jaut.2016.06.005
Wing JB, Sakaguchi S (2012) Multiple treg suppressive modules and their adaptability. Front Immunol 3:178. https://doi.org/10.3389/fimmu.2012.00178
Grant CR, Liberal R, Mieli-Vergani G et al (2015) Regulatory T-cells in autoimmune diseases: challenges, controversies and—yet—unanswered questions. Autoimmun Rev 14:105–116. https://doi.org/10.1016/j.autrev.2014.10.012
Sakaguchi S, Mikami N, Wing JB et al (2020) Regulatory T cells and human disease. Annu Rev Immunol 38:541–566. https://doi.org/10.1146/annurev-immunol-042718-041717
Miethke AG, Saxena V, Shivakumar P et al (2010) Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol 52:718–726. https://doi.org/10.1016/j.jhep.2009.12.027
Lages CS, Simmons J, Chougnet CA, Miethke AG (2012) Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 56:219–227. https://doi.org/10.1002/hep.25662
Tucker RM, Feldman AG, Fenner EK, Mack CL (2013) Regulatory T cells inhibit Th1 cell-mediated bile duct injury in murine biliary atresia. J Hepatol 59:790–796. https://doi.org/10.1016/j.jhep.2013.05.010
Li K, Zhang X, Yang L et al (2016) Foxp3 promoter methylation impairs suppressive function of regulatory T cells in biliary atresia. Am J Physiol Gastrointest Liver Physiol 311:G989–G997. https://doi.org/10.1152/ajpgi.00032.2016
Yang Y, Liu Y-J, Tang S-T et al (2013) Elevated Th17 cells accompanied by decreased regulatory T cells and cytokine environment in infants with biliary atresia. Pediatr Surg Int 29:1249–1260. https://doi.org/10.1007/s00383-013-3421-6
Saito T, Sakamoto A, Hatano M et al (2017) Systemic and local cytokine profile in biliary atresia. Eur J Pediatr Surg 27:280–287. https://doi.org/10.1055/s-0036-1592136
Hang S, Paik D, Yao L et al (2019) Bile acid metabolites control TH17 and treg cell differentiation. Nature 576:143–148. https://doi.org/10.1038/s41586-019-1785-z
Campbell C, McKenney PT, Konstantinovsky D et al (2020) Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581:475–479. https://doi.org/10.1038/s41586-020-2193-0
Katz SC, Ryan K, Ahmed N et al (2011) Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol 187:1150–1156. https://doi.org/10.4049/jimmunol.1004077
Weerasooriya VS, White FV, Shepherd RW (2004) Hepatic fibrosis and survival in biliary atresia. J Pediatr 144:123–125
Longhi MS, Mieli-Vergani G, Vergani D (2021) Regulatory T cells in autoimmune hepatitis: an updated overview. J Autoimmun 119:102619. https://doi.org/10.1016/j.jaut.2021.102619
Miyara M, Gorochov G, Ehrenstein M et al (2011) Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev 10:744–755. https://doi.org/10.1016/j.autrev.2011.05.004
Dominguez-Villar M, Hafler DA (2018) Regulatory T cells in autoimmune disease. Nat Immunol 19:665–673. https://doi.org/10.1038/s41590-018-0120-4
Long SA, Buckner JH (2011) CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol 187:2061–2066. https://doi.org/10.4049/jimmunol.1003224
Friedman SL (2004) Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1:98–105. https://doi.org/10.1038/ncpgasthep0055
Ward SM, Fox BC, Brown PJ et al (2007) Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol 47:316–324. https://doi.org/10.1016/j.jhep.2007.03.023
Hill R, Quaglia A, Hussain M et al (2015) Th-17 cells infiltrate the liver in human biliary atresia and are related to surgical outcome. J Pediatr Surg 50:1297–1303. https://doi.org/10.1016/j.jpedsurg.2015.02.005
Zhang S, Gang X, Yang S et al (2021) The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol 12:678355. https://doi.org/10.3389/fimmu.2021.678355
Lourenço JD, Ito JT, de Martins MA et al (2021) Th17/Treg imbalance in chronic obstructive pulmonary disease: clinical and experimental evidence. Front Immunol 12:804919. https://doi.org/10.3389/fimmu.2021.804919
Oita S, Saito T, Sakamoto A et al (2022) Frequency and function of circulating regulatory T-cells in biliary atresia. Pediatr Surg Int 39:23. https://doi.org/10.1007/s00383-022-05307-8
Mercadante ER, Lorenz UM (2016) Breaking free of control: how conventional T cells overcome regulatory T cell suppression. Front Immunol 7:193. https://doi.org/10.3389/fimmu.2016.00193
Buckner JH (2010) Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 10:849–859. https://doi.org/10.1038/nri2889
Acknowledgements
The authors thank Ms. Takana Motoyoshi (K. I. Stainer, Inc.) for their technical assistance.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers 15K10917 and 18K08535). None of the authors have any relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization: SO, TS; Methodology: SO, RH, J-II; Formal analysis and investigation: SO, RH, TF, YK; Writing—original draft preparation: SO; Writing—review and editing: KT, SK, AT, TH; Funding acquisition: TS; Supervision: J-II, TH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Graduate School of Medicine, Chiba University (approval number 908) and the Institutional Review Board of Chiba Children’s Hospital (2017–046).
Consent to participate and publish
Informed consent was obtained from all participants (or their parents) in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
383_2023_5547_MOESM1_ESM.tiff
Online Resource 1: Correlation between fibrosis score and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000 μm2 [mild (I): 26 ± 12; moderate (II): 34 ± 14; p = 0.22] (b) The mean number of CD4+ cells [mild (I): 11 ± 8.8; moderate (II): 2 4 ± 15; p = 0.05] (c) The mean number of CD4+FOXP3+ cells [mild (I): 3.2 ± 2.7; moderate (II): 8.0 ± 5.7; p = 0.05] (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) [mild (I): 0.30 ± 0.12; moderate (II): 0.32 ± 0.11; p = 0.66]. Supplementary file1 (TIFF 2209 KB)
383_2023_5547_MOESM2_ESM.tiff
Online Resource 2: Correlation between jaundice clearance and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000μm2 (failed jaundice clearance: 30 ± 18; achieved jaundice clearance: 38 ± 11) (b) The mean number of CD4+ cells (failed: 31 ± 18; achieved: 25 ± 11) (c) The mean number of CD4+FOXP3+ cells (failed: 12±7.6; achieved: 7.4±3.7) (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) (failed: 0.37 ± 0.033; achieved: 0.30 ± 0.11). Supplementary file2 (TIFF 2313 KB)
383_2023_5547_MOESM3_ESM.tiff
Online Resource 3: Correlation between outcome at 3 years and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000 μm2 [native liver: 35 ± 10; transplanted: 37 ± 18; p = 0.87] (b) The mean number of CD4+ cells [native liver: 21 ± 3.7; transplanted: 36 ± 16; p = 0.07] (c) The mean number of CD4+FOXP3+ cells [native liver: 6.2 ± 2.4; transplanted: 12 ± 5.5; p = 0.07] (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) [native liver: 0.30 ± 0.13; transplanted: 0.34 ± 0.051; p = 0.61]. Supplementary file3 (TIFF 2443 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oita, S., Saito, T., Hashimoto, R. et al. Frequency of infiltrating regulatory T-cells in the portal tract of biliary atresia. Pediatr Surg Int 39, 259 (2023). https://doi.org/10.1007/s00383-023-05547-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05547-2