Abstract
Purpose
Among factors promoting intestinal adaptation after bowel resection, dietary fatty acids have a special role. The purpose of the present study was to evaluate the effects of palmitic acid (PA) on early intestinal adaptation in rats with short bowel syndrome (SBS).
Materials
Male Sprague–Dawley rats underwent either a bowel transection with re-anastomosis (sham rats) or 75% small bowel resection (SBS rats). Animals were randomly assigned to one of four groups: sham rats fed normal chow (sham-NC); SBS rats fed NC (SBS-NC), SBS rats fed high palmitic acid diet (SBS-HPA), and SBS rats fed low palmitic acid diet (SBS-LPA). Rats were sacrificed on day 14. Parameters of intestinal adaptation, overall bowel and mucosal weight, mucosal DNA and protein, villus height and crypt depth, cell proliferation and apoptosis were determined at sacrifice. RT-PCR and Western blotting were used to determine the level of bax and bcl-2 mRNA and protein (parameters of apoptosis), and ERK protein levels (parameter of proliferation). Statistical analysis was performed using Kruskal–Wallis test followed by post hoc test for multiple comparisons with P values of less than 0.05 considered statistically significant.
Results
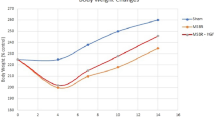
SBS-HFD rats demonstrated higher bowel and mucosal weight, mucosal DNA and protein in ileum, while deprivation of PA (SBS-LPA) inhibited intestinal re-growth both in jejunum and ileum compared to SBS-NC rats. A significant up-regulation of ERK protein coincided with increased cell proliferation in SBS-HFD rats (vs. SBS-NC). Also, the initial decreased levels of apoptosis corresponded with the early decrease in bax and increase in bcl-2 at both mRNA and protein levels.
Conclusion
Early exposure to HPA both augments and accelerates structural bowel adaptation in a rat model of SBS. Increased cell proliferation and decreased cell apoptosis may be responsible for this effect. Deprivation of PA in the diet inhibits intestinal re-growth.





Similar content being viewed by others
References
Biller JA (1987) Short bowel syndrome. In: Grand RI, Sutphen JL, Dietz WH (eds) Pediatric nutrition. Theory and practice. Butterworth Publishers, Stoneham, pp 481–487
Wesser E (1979) Nutritional aspects of malabsorption: short gut adaptation. Am J Med 67:1014–1019
Levine GM, Deren JJ, Yezdimir E (1976) Small-bowel resection: oral intake is the stimulus for hyperplasia. Am J Dig Dis 21:542–546
Vanderhoof JA (1996) Short bowel syndrome. Neonatal Gastroenterol 23:377–386
Hart MH, Grandjean CJ, Park JHY, Erdman SH, Vanderhoof JA (1988) Essential fatty acid deficiency and postresection mucosal adaptation in the rats. Gastroenterology 94:682–687
Sukhotnik I, Mor-Vaknin N, Drongowski RA et al (2004) Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int 20(4):235–239
Sukhotnik I, Shiloni E, Krausz MM, Yakirevich E, Sabo E, Mogilner J, Coran AG, Mac Harmon C (2003) Low fat diet impairs postresection intestinal adaptation in a rat model of short bowel syndrome. J Pediatr Surg 38:1182–1187
Park JHY, Grandjean CJ, Hart MH, Vanderhoof JA (1989) Effects of dietary linoleic acid on mucosal adaptation after small bowel resection. Digestion 44:57–65
Frémy E (1842) Memoire sur les produits de la saponification de l’huile de palme, Journal de Pharmacie et de Chimie XII, p 757
Sukhotnik I, Yakirevich E, Coran AG et al (2002) Transforming growth factor-alpha increases enterocyte proliferation, decreases apoptosis and stimulates intestinal adaptation in a rat model of short bowel syndrome. J Surg Res 108:235–242
Chromozinski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15:532–536
Grey VL, Garofalo C, Greenberg GR, Morin CL (1984) The adaptation of the small intestine after resection in response to free fatty acids. Am J Clin Nutr 40:1235–1242
Maxton DG, Cynk EU, Jenkins AP, Thompson RPH (1989) Effect of dietary fat on the small intestinal mucosa. Gut 30:1252–1255
Singh A, Balint JA, Edmonds RH, Rodgers JB (1972) Adaptive changes of the small intestine in response to a high fat diet. Biochim Biophys Acta 260:708–715
Kollman KA, Lien EL, Vanderhoof JA (1999) Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr 28:41–45
Bracco U (1994) Effect of triglyceride structure on fat absorption. Am J Clin Nutr 60(Suppl):S1002–S1009
Palacios A, Catalá A (1991) The palmitic acid binding properties of cytosolic proteins located in the villus and crypt zones of bovine intestinal mucosa. Vet Res Commun 15:437–442
Artmann A, Petersen G, Hellgren LI et al (2008) Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta 1781:200–212
Kollman KA, Lien EL, Vanderhoof (1999) Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastr Nutr 28:41–45
Bowen JM, Gibson RJ, Cummins AG, Keefe DM (2006) Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 14:713–731
Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391:496–499
Stern LE, Falcone RA, Kemp CJ, Stuart LA, Erwin CR, Warner BW (2000) Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis. J Gastrointest Surg 4:93–100
Falcone RA, Stern LE, Kemp CJ, Shin CE, Erwin CR, Warner BW (1999) Apoptosis and the pattern of DNase I expression following massive small bowel resection. J Surg Res 84:218–222
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Sukhotnik and L. Hayari contributed equally to the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Sukhotnik, I., Hayari, L., Bashenko, Y. et al. Dietary palmitic acid modulates intestinal re-growth after massive small bowel resection in a rat. Pediatr Surg Int 24, 1313–1321 (2008). https://doi.org/10.1007/s00383-008-2272-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2272-z