Abstract
Climate change scenarios predict more frequent and intense drought periods for 2071 to 2100 for many regions of the world including Austria. Current and predicted lower precipitation scenarios were simulated at a lysimeter station containing a fertile and less fertile agricultural soil for 9 years. 13C and 15N-labeled green manure was added in year 8 with the aim to analyze how the predicted precipitation regime affects soil fauna and microbial groups and consequently nitrogen (N) and carbon (C) cycling. Among the investigated mesofauna (collembola and oribatida), the abundance and biodiversity of oribatida was significantly reduced by drought, possibly because they mainly represent K-strategist species with low mobility and consequently the need to adapt to long-term adverse environmental conditions. Microbial community composition and microbial biomass, investigated by phospholipid fatty acid (PLFA) analysis, was indistinguishable between the current and the predicted precipitation scenarios. Nonetheless, soil 13C-CO2 emissions and soil water 15N-NO3 data revealed decelerated mineralization of green manure under reduced precipitation in the first 2 weeks, but no effects were observed on soil C sequestration or on 13C incorporation into microbial PLFAs in the following 1.2 years. We found that over a 1-year time period, decomposition was rather driven by plant residue availability than water limitation of microorganisms in the investigated agroecosystem. In contrast, N2O emissions were significantly reduced under drought, and green manure derived 15N accumulated in the soil under drought, which might necessitate the adjustment of future fertilization regimes. The impacts of reduced precipitation and drought were less pronounced in the more fertile agricultural soil, due to its greater buffering capacity in terms of water storage and organic matter and nutrient availability.
Similar content being viewed by others
Introduction
Climate models predict an increase in average air temperatures, and a shift in precipitation patterns, resulting in more intense and frequent summer heat-drought waves, accompanied by heavier intermittent rainfall events in many regions of the world, including Austria (APCC 2014; IPCC 2021; Thaler et al. 2021). A drought event encompasses a soil water deficit, which exerts various stress effects on plants (Crocetti et al. 2020) and soil microbes (Schimel 2018), consequently impacting the C and N cycle.
Drought impacts belowground microbial communities by two factors: the direct water stress (osmotic stress) associated with drought, and indirect impacts such as reduced plant C input and reduced substrate access via diffusion, due to the lack of water as a transport medium for nutrients and enzymes (Schimel 2018). Soil microorganisms have a relatively low water potential optimum (0.3 MPa) (Lennon et al. 2012) and can withstand water potentials of around −14 MPa (Manzoni et al. 2012). Consequently, Schimel (2018) concluded that many drought effects observed for soil microbial communities are related to nutrient/resource limitations. Nutrient availability depends on its concentration, accessibility via diffusion processes, and retention and stabilization processes, such as sorption and complexation, which are determined by the physicochemical properties of the soil. Hence, soil properties will indirectly impact the resistance and resilience of soil microbial communities (Bardgett and Caruso 2020) and thereby microbial biogeochemical processes and nutrient availability to crops. Soils containing high amounts of potentially available nutrients might be more resilient to changing climate.
Additionally, the observed impact of drought on soil microbiology also depends on temporal factors. At high time resolution (hourly to daily) across a drying-rewetting cycle, the differences in drought response patterns become clear and are linked to different functional and broad phylogenetic groups (Bardgett et al. 2001). Short-term impacts are strongly related to the strategies of soil organisms to deal with drought: some organisms are resistant to drought stress, while others recover quickly (resilient), which can be related to specific microbial groups (De Vries and Shade 2013). For instance, K-strategists such as fungi and Gram-positive bacteria exhibit a high resource use efficiency but are slow growing, being highly drought-resistant, but showing a low drought-resilience. On the contrary, r-strategists, such as Gram-negative bacteria, show low drought-resistance but can reproduce quickly and therefore show high resilience. To date, it is unclear if this holds true in the long-term, when K-strategists have enough time to adapt to the changing climatic conditions.
The activity of microorganisms is affected and top-down controlled by the mesofauna, which plays an important role in the decomposer food web, either by direct decomposition of leaf and root litter, and indirectly by promoting microbial litter degradation through fragmentation and fecal production, or by grazing on primary degraders and their predators (Seastedt 1984; Sackett et al. 2010). Mesofauna includes microarthropods, with collembola (springtails) and acari (mites) contributing the largest fraction to the soil mesofaunal biomass in temperate climates and being useful bio-indicators (Gardi et al. 2009). Drought will likely decrease the abundance of both acari and collembola, with collembola responding stronger to precipitation changes than acari (Blankinship et al. 2011). These findings from a meta-analysis were confirmed in more recent publications, which in addition also documented decreased microarthropod biomass and species richness and altered microarthropod species composition (Kardol et al. 2011; Xu et al. 2012; Vestergård et al. 2015; Aupic-Samain et al. 2021; Meyer et al. 2021). Generally, microarthropods benefit from high organic matter input and an aggregated soil structure, which serves as a habitat and enables migration to moister microhabitats as a drought avoidance strategy (Meyer et al. 2021). The climate history, e.g., recurrent drought, may lead to adaptation of the soil microbial and faunal communities, thereby assisting in maintaining the multifunctionality of soils and increasing their resilience (Preece et al. 2019; Brangarí et al. 2021; Canarini et al. 2021).
The drought response patterns of plants and soil biota will reciprocally interact with soil C and N dynamics. Even though plants positively affect the resilience of soil biota (De Vries and Shade 2013), plants themselves respond to drought stress, by reducing photosynthetic C fixation, modifying their C allocation, and altering their root exudate and litter quantity and quality, all of which can strongly impact soil microbial (Naylor and Coleman-Derr 2018; Pugnaire et al. 2019) and microarthropod activity and function (Seeber et al. 2012; Santonja et al. 2017). During prolonged and more intense drought events, the ecosystem C cycle is likely to slow down, due to lower plant C input and diminished plant residue mineralization (Wu et al. 2011). Subsequent rewetting causes a pulse of C mineralization and soil CO2 release (Birch 1958). Carbon mineralization during rewetting depends on (1) the total organic matter content as the potentially available C source and the bioavailability of labile C regulated by sorption versus desorption processes, i.e., parameters depending on the soil characteristics and (2) the intensity of prior drought, and the legacy of previous drying/rewetting cycles or of other physical disturbances (e.g., tillage), i.e., factors depending on climate and land management (Borken and Matzner 2009; Canarini et al. 2017; Schimel 2018). Accordingly, meta-analyses have shown both effects, i.e., an increase in soil C mineralization (Deng et al. 2021) and a decrease thereof (Borken and Matzner 2009; Wu et al. 2011; Deng et al. 2021). Considering the ecosystem N cycle, a reduction in plant N uptake and an accumulation of inorganic and organic N in the soil were reported (Deng et al. 2021). Under drought, organic N mineralization can exceed N uptake by plants, leading to the accumulation of NH4+ in the soil. In dry soils, NH4+ diffusion towards nitrifiers is strongly restricted and nitrification/denitrification therefore slows down, consequently reducing the amount of reactive N and N2O gaseous losses (Homyak et al. 2017; Schimel 2018; Li et al. 2020).
It is commonly agreed that ecosystem resistance and resilience to drought increase with increasing biodiversity due to the asynchrony of species’ responses (tolerance versus recovery) and changes in the food web interactions (Bardgett and Caruso 2020). Processes, which can be mediated by many species, such as plant residue mineralization, are likely to be less susceptible to drought than for example denitrification (Homyak et al. 2017). Vice versa, the impact of drought might be more visible on a species level than on a group level of low taxonomic resolution. In agroecosystems, biodiversity is lower than in natural ecosystems (Postma-Blaauw et al. 2010; Tsiafouli et al. 2015). Furthermore, long-term cultivated agricultural soils become impoverished in soil organic C content and exhibit a deterioration of the soil structure due to tillage. Low biodiversity and deteriorated soil characteristics might especially impair the ecosystem functioning under drought, particularly in non-fertile agricultural soils. Nonetheless, long-term field studies investigating the impact of more frequent and intense periods of drought waves on the C and N cycle in agroecosystems are still rare.
A stable isotope labeling experiment was conducted on a lysimeter site, which has been subject to simulated frequent and intense drought events for 9 years to enable the investigation of a fully adapted soil mesofaunal and microbial community. We used 13C and 15N-labeled green manure in year 8 to enable the study of the (un) coupling of ecosystem C and N fluxes in an atmosphere-plant-soil-microbe system. Mesofauna was monitored at a species level to better observe drought-related adaptations. Microorganisms were targeted using phospholipid fatty acid (PLFA) analysis because coupling with C stable isotope measurements provides linkage between taxonomic and functional information (Watzinger 2015). We hypothesize that soil characteristics will influence the C and N cycle of newly added plant residues by affecting both bioavailability of nutrients and potential microbial activity. This study aims at (1) identifying the impact of long-term drought on the soil microbial community and mesofauna; (2) monitoring the alteration in the C and N cycle of newly added plant residues; and (3) defining the magnitude of response of fertile versus less fertile agricultural soil.
Materials and methods
Experimental set-up
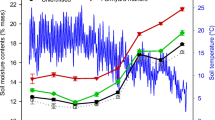
The AGES lysimeter facility consists of 18 filled gravitation-lysimeters representing the three main soil types of the Pannonian region in Austria, namely calcaric Phaeozem (Ps), calcic Chernozem (Ch), and gleyic Phaeozem (Pg) (Gerzabek and Krenn 1996; Stenitzer and Hösch 2005). The gleyic Phaeozem, a groundwater influenced soil type, had become dry and experienced unnatural shrinking and crack formation and was therefore excluded from the isotope labeling experiment. The main soil chemical and physical properties of the upper 10 cm were re-determined in 2019 and are presented in the Supplementary Information Table 1. Rainfall has been simulated with an overhead irrigation system since 2011 (Tataw et al. 2014, 2016). For the “current” scenario, half of the lysimeters were irrigated according to current precipitation patterns, as calculated by the 30-year-mean of rainfall (amount and distribution) in the nearby village Großenzersdorf (517 mm). The other half of the lysimeters in the “predicted” scenario were watered according to the precipitation predicted for the period 2071 to 2100 in the Pannonian region, simulating drought periods and heavy rain events from 2011 until 2017 (436 mm) (Tataw et al. 2016). From 2018 onwards, an adapted and improved model, the historical (1961 to 2000) and the hot and dry model scenario presented in Seidl et al. (2019), was used to calculate “current” and “predicted” scenarios (531 mm and 386 mm precipitation per annum). The regional climate change scenarios are based on four selected regional climate model scenarios from Euro-Cordex (Jacob et al. 2014) out of the ensemble ÖKS 15 (Leuprecht et al. 2016), derived from different global and regional climate models and were forced with the emission scenarios RCP 4.5 and RCP 8.5. The ÖKS15 ensemble provides bias-corrected and localized (1 km grid) scenarios for daily precipitation. Bias correction was done using quantile mapping (Switanek et al. 2017). The lysimeter station was covered by a plastic tunnel during the vegetative period and remained uncovered in winter, when it received ambient precipitation. Figure 1 depicts the period of coverage (simulated precipitation) as well as the mean daily temperature and the amount of rainfall and irrigation from 2017 to 2019 based on precipitation scenarios.
Period of cultivation, plant leaf, water, soil and gas, and mesofauna sampling dates are illustrated on the top. Daily mean air temperatures and accumulated rainfall/irrigation under the current (grey line) and predicted precipitation scenario (black line) are shown for the years 2017, 2018, and 2019. The dashed straight line indicates the time of simulated precipitation. The amount and timing of NH4NO3 fertilization is illustrated as bars. Black bars indicate the amount of N fertilizer applied to the Phaeozem and grey bars indicate the additional fertilizer applied to the Chernozem. The date of 15N and 13C green manuring (mustard) is indicated by an arrow
The crop rotation and crop residue management throughout the experiment followed the common agricultural practices in the study region. In 2017, spring wheat (Triticum turgidum subsp. durum (Desf.) Husn) cf. Floradur was grown followed by white mustard (Sinapis alba L.) as a catch crop. In spring 2018, spring barley (Hordeum vulgare L. cf. Cerbinetta) was sown followed by winter wheat (Triticum aestivum L.) cf Capo in autumn 2018 (Fig. 1). Before sowing and after the harvest, the soil was manually tilled. Fertilizer was applied according to Austrian standard recommendations (BMLFUW 2017) (Fig. 1). Visual controls for pest infestations were done regularly to identify potential insect pests and to allow for appropriate measures to be taken. No significant insect infestations were detected for the duration of the experiments described. However, weeds were relatively abundant and were removed manually (omitting the use of pesticides), to avoid potential negative effects on the soil microbial community.
Stable isotope labeling experiment
A 13C and 15N labeling experiment was conducted to assess the effect of predicted precipitation on soil C and N mineralization of green manure and nutrient cycling. Isotopically labeled mustard residues were incorporated into the upper 10 cm of topsoil in a small, framed segment (0.25 m2) of the lysimeters in spring 2018. Labeling of mustard was done in a controlled laboratory environment in the greenhouse by watering pre-seeded mustard plants with a 15N-labeled micronutrient solution and by pulse labeling the plants in a gas tight chamber filled with 13C-labeled CO2. Final delta values of 13C and 15N-labeled mustard mulch were 65 ± 12 ‰ and 170 ± 10 ‰, respectively. Soil sampling was done repeatedly to a depth of 10 cm using a metal soil corer (0.6 cm diameter) before labeling, 6 hours, 2, 4, 9, 21, 32, and 72 days, and 0.9, 1.1, and 1.2 years after labeling (Fig. 1). Seepage water only occurred in some lysimeters during spring and after a simulated heavy rainfall event (60 mm) in 2018 (day 16). Then, soil water samples were taken at different soil depths from 10 cm down to 250 cm depth from preinstalled suction cups in the lysimeters and by collecting drainage water at the bottom of the lysimeters. Gas samples were taken on the days of soil sampling in the field to gain insight into soil respiration dynamics (CO2 measurements) and N2O fluxes. Additionally, N2O emissions were measured on the following 2 days after the heavy rainfall event and after N fertilization. Leaves were sampled during growth and at crop harvest (days 21, 32, 72, 105 and years 0.9, 1.1, and 1.2) for elemental and isotopic analyses in the laboratory (Fig. 1).
Soil water content was determined gravimetrically after drying fresh soil aliquots at 105 °C for 24 h. Plant and bulk soil C and N content and isotopic composition were analyzed by Elemental Analyzer – Isotope Ratio Mass Spectrometry (EA-IRMS; elemental analyzer coupled to Delta V Advantage, Thermo Scientific, Germany). Soil inorganic C was removed beforehand via HCl fumigation following Harris et al. (2001). Soil NO3 and NH4 were extracted from fresh soil samples using 0.5 M K2SO4 using a micro-diffusion method (Heiling et al. 2006): NH4 and NO3 were sequentially captured into acid traps that were then analyzed for their N content and 15N signature by EA-IRMS. Microbial biomass was determined by pre-extraction with 0.5 M K2SO4 followed by liquid chloroform — K2SO4 extraction (Setia et al. 2012). Microbial biomass N content and δ15N values were measured by purge-and-trap IRMS (PT-IRMS, Gasbench II headspace analyzer coupled to Delta V Advantage, Thermo Fisher, Bremen, Germany) following the procedure of Lachouani et al. (2010). Nitrate concentrations of soil water were determined colorimetrically by the VCl3/Griess assay (Hood-Nowotny et al. 2010). Delta 15N values of nitrate in soil water were analyzed by purge-and-trap IRMS as mentioned for microbial δ15N determination. Phospholipid-derived fatty acids (PLFAs), the primary lipids in cellular membranes of soil microbiota, were extracted and the concentration and δ13C was measured via Gas Chromatograph - Isotope Ratio Mass Spectrometer (GC-IRMS; HP 5890 Series II Gas Chromatograph coupled to a Delta S, Finnigan, Germany) (Watzinger 2015; Watzinger and Hood-Nowotny 2019).
Soil N2O fluxes were measured on all lysimeter plots by laser spectroscopy (Los Gatos Research (LGR) Isotopic Off-Axis Integrated Cavity Output N2O analyzer, Model 914-0027) connected to a gastight chamber with a measurement sequence of 30 min. Additionally, air samples were taken from each lysimeter at 4 sampling times (0, 10, 20, 30 min), and concentrations and δ13C values of CO2 were determined by gas chromatography (GC)-IRMS (Thermo Fisher Trace GC Ultra connected to IRMS, Thermo Fisher Delta V Advantage) (Leitner et al. 2020). The Keeling plot approach was used to determine δ13C values of soil CO2 efflux for each lysimeter (Keeling 1958), and fluxes of soil CO2 emissions were calculated for each sampling time.
Low mesofauna biomass prevented the measurement of its stable isotope ratio. Therefore, the sampling dates were optimized to gain an annual overview on their abundance. Following this, collembola (springtails) and oribatida (beetle mites) were sampled three times a year: in spring, summer, and autumn (Fig. 1). On each date, five soil cores (56 × 56 mm × 100 mm depth) were taken from each lysimeter, transferred to the laboratory, and extracted in a Berlese-Tullgren apparatus. The five subsamples of each lysimeter were pooled, and the collembola and oribatida were manually picked from the debris. The collembola were counted and analyzed as a group, and the oribatida were identified to species level and counted.
Statistical analysis
Two- or 3-way ANOVA type III sum of squares analyses using soil type, precipitation treatment, and/or time as main factors were run, and Bonferroni post hoc tests were conducted. Generally, no transformation of the raw data was necessary to meet the assumptions of normality and variance homogeneity. Those very few data sets being transformed are indicated in the respective tables of the Supplementary Information. Mesofauna abundance data were log10-transformed and analyzed using a linear mixed effects model, with soil and precipitation as fixed factors and sampling date as a random factor. In addition, principal component analyses were conducted. Prior to principal component analysis, abundances were Hellinger transformed to reduce the influence of dominant species. For statistical analysis, the R package Jamovi was used (R Core team 2020; The jamovi project 2021; Gallucci 2019).
Results and discussion
Responses of the soil mesofauna (collembola and oribatida) to drought
In total, 9023 collembola and 3138 oribatida specimens were collected per m2. The abundance of collembola was dominated by one excessively large sample in spring 2018. Here, one lysimeter accounted for 2751 individuals, which is approximately 30% of all individuals found. Collembolan abundance was significantly affected by soil type, but not by the precipitation scenario (Supplementary Information Table 2). In contrast to the collembola, we found consistent and significant effects of both, soil type and precipitation regime, on the total number of oribatid mites (Fig. 2, Supplementary Information Table 2). In total, Chernozem contained approximately 3 times more individuals of oribatid mites than the Phaeozem, and the current precipitation treatment 1.6 times more individuals than the predicted precipitation scenario. The latter was in the range of the difference in plant biomass production between current and predicted precipitation scenarios.
Collembola and oribatida abundances were similar to those reported in dry and conventional agroecosystems (Meyer et al. 2021), but strongly reduced compared to those reported in natural ecosystems (Cole et al. 2006; Holmstrup et al. 2017; Peguero et al. 2021). However, in contrast to our findings, other studies reported that collembola are more sensitive to drought than oribatida (Holmstrup et al. 2013; Meyer et al. 2021). Both collembola and oribatida are detritivore organisms, though they also graze on the soil microbial community (Behan-Pelletier 1999; Chahartaghi et al. 2005). The overall amount of plant residues input was possibly higher in the Chernozem and under the current precipitation scenario, due to higher plant growth (unpublished data), which was most probably the main positive driver of mesofaunal abundance (Blankinship et al. 2011). Lower soil bulk density, higher soil moisture, and a better soil structure and aggregate stability might have further promoted mesofaunal community abundance in the Chernozem. Oribatid mites are considered K-strategists, with a slow development and a long life cycle, and therefore are expected to respond slowly to long term degradation of their microhabitat (Behan-Pelletier 1999). Accordingly, their abundance and biodiversity decreased after 9 years of the predicted precipitation scenario in this experiment. In contrast, collembola are r-strategists, recover quickly, and are more mobile than oribatida (Alvarez et al. 1999; Lindberg and Bengtsson 2006; Pollierer and Scheu 2017). Hence, collembola might be able to profit from short-term improvements in habitat conditions due to rainfall events and/or escape desiccation by downward migration, while oribatida reflected the impact of long-term habitat decline due to drought more sensitively.
Only nine species of oribatida were collected (Supplementary Information Table 3), which is a much lower biodiversity than observed in the previous investigations in 2011–2013 (26 species; unpublished data). All species found in 2011–2013 were reported 6 years later. Hence, species richness had dropped by 66%, irrespective of precipitation treatment. This was likely related to the disruption of soil aggregates and hence the deterioration of their microhabitat during 6 years of irrigation. In 2017–2019, the Shannon index was significantly higher under the current than under the predicted precipitation regime, with a significant precipitation/soil interaction term (Supplementary Information Table 3). This was visualized by a principal component plot (Supplementary Information Fig. 2), where the oribatid mite assemblages of the two precipitation scenarios were clearly separated between the sandy Phaeozem, but not for the Chernozem. These findings are similar to that of Meyer et al. (2021), who reported buffering of negative effects of drought on oribatida communities due to higher soil organic C content, associated with a higher soil water content and improved soil structure.
Impact of drought on soil microbial PLFAs
The gravimetric soil water content determined during soil sampling in the top 10 cm of soil was not significantly different between current and predicted precipitation scenarios, which can be attributed to the different precipitation regimes (Table 2, Supplementary Information Fig. 2). The soil sampling dates were fixed, meaning that the predicted scenario could have received irrigation the day before sampling while the current scenario did not. Nonetheless, the soil water content approached the wilting point (−1.5 MPa), but was still far away from the water potential suggested to cease soil microbial activity (−14 MPa) (Manzoni et al. 2012).
Single soil microbial PLFAs were unaffected by the different precipitation scenarios, while PLFA contents were affected by soil type and sampling time. Drought has been documented to alter the soil PLFA patterns and increase the fungi to bacteria and gram+ to gram- bacteria ratios (De Vries and Shade 2013). The investigated soils had developed under continental climate, with low precipitation (600 mm) and high mean annual temperature, and were used for agricultural purposes for decades. The current labeling experiment began 7 years after both precipitation scenarios had been subjected to many drying/rewetting cycles and intense rainfall events, resulting in soil disaggregation and soil structure loss (visual inspection), which may have released potentially bioavailable organic C (Borken and Matzner 2009). Drought, loss of bioavailable C and microhabitat deterioration had likely fostered the development of a resistant soil microbial community in both, the current and the predicted precipitation scenarios (Ren et al. 2017; Leizeaga et al. 2022). As an indicator of microbial resistance, the fungi to bacteria ratios were high (Table 1), especially when considering that bacteria generally dominate in agricultural soils (high N supply, tillage) (Bardgett and McAlister 1999; De Vries et al. 2006). In addition, we speculate that a combination of low number of replicates (n=3), field-like conditions with a high variation of applied “precipitation” events per lysimeter, and a sampling regime that did not take into consideration the various drying and rewetting cycles, caused high data variability, hindering the statistical evaluation of small eventual shift in microbial PLFA pattern under the predicted precipitation regime.
Nonetheless, and as expected, due to the higher soil water availability and organic matter content, microbial biomass was higher in the Chernozem (Tables 1 and 2), due to a higher PLFA-based soil bacterial biomass. The presence of fungi was unaffected by soil type, again confirming their resistance to possible nutrient and water deficiencies (Canarini et al. 2017). PLFA patterns were also affected by sampling time, but this appeared not to be driven by the addition of the green manure in April 2018. The addition of significant amounts of plant residues usually rapidly increases the soil microbial biomass (Stemmer et al. 2007; Djukic et al. 2013). However, in our experiment, the amount of mustard added was low (0.2 mg gsoil−1) and did not lead to a significant increase in the soil C and/or microbial biomass.
Carbon mineralization in soils and identification of decomposer soil microorganisms
The short-term microbial mineralization of 13C-labeled mustard as detected by soil CO2 emissions and isotope tracing was significantly decreased under the predicted precipitation scenario (Table 2, Supplementary Information Fig. 3). Total soil CO2 emissions decreased by 13% and 25% in the Phaeozem and Chernozem respectively, which aligns with previous studies of impacts of drought on soil microbial respiration (−16% and −12%) and litter decomposition (−13%) (Wu et al. 2011; Canarini et al. 2017; Deng et al. 2021). Calculating the mustard-derived CO2 emissions revealed that drought only affected the mineralization of the newly added green manure, and that its flux was decreasing in the Chernozem, due to higher microbial activities rapidly depleting the added labeled manure. Despite the observed short-term difference in green manure mineralization between current and predicted precipitation scenarios, neither the content nor the δ13C values of the overall soil organic C pool were affected by precipitation treatment, indicating that native organic C mineralization was not affected, and that the C input/output was balanced during several months in this agroecosystem.
The microbial anabolism of labeled mustard into PLFAs started within hours in all microbial groups after mustard addition, likely due to the presence of readily available and widely usable labile mustard C compounds. However, the extraordinarily high initial δ13C values of the PLFAs 16:0, 18:0, 18:2ω6,9c, and 18:1ω9c were attributed by 27%, 15%, 13%, and 15% to the addition of labeled mustard as green manure. The input of those fatty acids by plants has already been documented and discussed critically (Zelles 1999; Frostegård et al. 2011; Joergensen 2022). The δ13C values of bacterial PLFAs were higher in the Phaeozem likely due to its lower soil organic C content and thus the preferred biodegradation of the newly added green manure. In contrast to bacteria, saprophytic fungi (16:0, 18:0, and 18:2ω6,9c) incorporated more 13C from green manure in the Chernozem than in the Phaeozem (Supplementary Information Fig. 4 and Supplementary Information Table 4). The Chernozem might have retained added organic residues better than the Phaeozem, due to its loamy texture and higher organic content. Fungi have the ability to access these adsorbed and complex organic residues by hyphal growth and by the excretion of extracellular enzymes (De Boer et al. 2005). Additionally, fungi incorporated more than double the amount of 13C-labeled green manure into their PLFAs than bacteria, while actinobacteria and arbuscular mycorrhizal fungi incorporated only half of this amount. As both, higher and lower C use efficiencies in fungi than in bacteria have been reported (Strickland and Rousk 2010; Soares and Rousk 2019), our results indicate that fungi were more active than bacteria in green manure decomposition and utilization in this agroecosystem. In the second year, mustard was still degraded (primarily by fungi) and/or the label was recycled within the microbial community. Our results therefore confirm former studies, i.e., that (1) a broad range of soil microorganisms quickly utilize plant residues (a functional redundant process) (Schimel and Schaeffer 2012), that (2) fungi have a higher C incorporation/mineralization potential than bacteria, and incorporate and degrade plant residues for several weeks/month (Rousk and Bååth 2007), and (3) that microbial communities in low soil organic matter and sandy textured soils anabolized more C, indicating C driven limitation rather than drought limitation of microbial functions and processes (Schimel 2018). Most importantly, C incorporation by microbial communities was not affected by the precipitation regime. Carbon limitation, the presence of drought adapted microorganisms, and decoupling of sampling and the drying/rewetting cycle had likely ceased the impact of the precipitation scenario.
Soil nitrogen cycling
Bulk soil N was significantly higher in the Chernozem than in the Phaeozem, reflecting the generally higher soil fertility of the Chernozem (Table 3). This was also the case for soil nitrate, but not for soil ammonium, which did not significantly differ between soils. However, the precipitation treatment affected none of the measured soil N pool sizes (bulk N, available ammonium and nitrate). Only the bulk soil δ15N values were higher under drought stress and especially in summer on the day of harvest (72 days after 15N green manuring), indicating one or several of the subsequent processes: slower plant residue decomposition, less uptake of mineralized mustard N via plants, or fewer N losses through leaching or gaseous N losses.
N mineralization and nitrification
In agreement with microbial biomass C data, soil microbial biomass N contents and δ15N values were not affected by the precipitation treatment (Table 3). Initially, retarded soil respiration had not led to reduced microbial N, as observed by van Meeteren et al. (2008) and Deng et al. (2021). The available NH4 and NO3 concentrations were not impacted either, which is in contrast to other studies, which reported inorganic N accumulation due to continuous N mineralization and nitrification but decreased plant N uptake under drought (Evans and Burke 2013; Homyak et al. 2017), or reduced N mineralization (−6%) and nitrification (−14%) but accumulation of dissolved organic N (+33%) (Deng et al. 2021). In addition, the δ15N value of available soil NO3 was not significantly impacted by drought. Since neither the concentration nor the δ15N values of microbial N and inorganic N were impacted by the precipitation regime, we concluded that the related processes of green manure mineralization/nitrification were not affected or were balanced by consumption processes such as plant N uptake or denitrification throughout 1.2 years.
Nitrate leaching
Unfortunately, the determination of nitrate leaching was hampered by the fact that only few soil water samples could be collected by suction cups from the lysimeters or their seepage water for soil water analysis, which made statistical analyses impossible. However, on the day of the heavy rainfall event (day 16), control treatments of both soil types had slightly higher δ15N-NO3 values and nitrate concentrations at 10 cm sampling depth, which suggests a slower decomposition and reduced nitrification under drought (Supplementary Information Table 5).
N2O emissions
Measured N2O emission data were generally low in comparison to those measured and modeled for comparable agricultural settings (Wang et al. 2021). Nonetheless, the drought treatment significantly reduced N2O emissions over the period of 2 years (2018, 2019) (Fig. 3, Supplementary Information Table 6). Most important factors controlling N2O emissions from soils comprise the availability of substrate (N) and soil water and oxygen content. Accordingly, the inorganic fertilizer addition (day 29) strongly increased soil N2O emissions (Fig. 3). The heavy rainfall event increased N2O emissions more under drought and in the sandy soil, i.e., under water limiting situations, while NH4NO3 fertilization had more impact on the soil N2O emissions of the Chernozem, and under current precipitation scenarios, indicating that this process was triggered by enhanced inorganic N availability (Fig. 3). Slightly smaller elevated N2O emissions as a consequence of a water pulse after prolonged drought were already described by others, e.g., Evans and Burke (2013) and were attributed to inorganic N accumulation during the drought period, followed by nitrification (Homyak et al. 2017; Schimel 2018). In our study, soil NH4 concentrations were not elevated and N2O emissions increased slowly after the heavy rainfall event. Heavy rainfall might have triggered several processes, e.g., green manure mineralization, nitrification (detected as increased δ15N-NO3 values on day 21), and possibly denitrification. We observed soil N2O emission reductions of 20 to 50% in the sandy Phaeozem and Chernozem under the predicted precipitation regime, excluding heavy rainfall emission data. These data are in concordance with studies that found N2O emission reductions of 31 to 53% under drought (Homyak et al. 2017; Li et al. 2020). Excluding periods < 4 days after fertilization, drought-related reductions of N2O emissions were lower (5% and 20% in the sandy Phaeozem and Chernozem, respectively) because microorganisms produced relatively high peak N2O emissions after fertilizer addition in the current compared to the predicted precipitation scenario. Concerning the prevailing microbial N2O production processes, the low water filled pore space (WFPS) of 5–30 to 10–35 Vol% in the sandy Phaeozem and Chernozem, respectively, points towards aerobic denitrification and nitrification (Bateman and Baggs 2005). Homyak et al. (2017) concluded that denitrification was more sensitive to drought compared to microbial processes that control soil N supply, reflecting the narrower phylogenetic distribution of denitrifiers compared to N mineralizing microorganisms. Denitrifiers require wetter conditions to maintain high rates of metabolic activity (Lennon et al. 2012). Nitrification might have been limited by the reduced diffusion of NH4+. Diffusion of NH4+ decreases at a water potential of less than −0.6 MPa (Stark and Firestone 1995), which translates to 23 and 40% WFPS in the sandy Phaeozem and Chernozem, respectively, a situation commonly encountered in our study.
Arithmetic mean and standard deviation (n=3) of the N2O emissions in sandy Phaeozem (Ps) and Chernozem (Ch) under the predicted and current precipitation scenario. A heavy rainfall event was simulated on day 16; N fertilization was performed on day 29. Data of days 17, 18, 29, and 30 are pseudoreplicate measurements and illustrated as triangles (1 plot per treatment, measured 3 times a day)
Analyses of collected crop leaves and harvested crops (straw and grain) underpin these findings and additionally allow conclusions on the pattern of green manure decomposition. Leaf N contents were higher in the Chernozem, most probably because of the inherent higher fertility of this soil type, but it was unaffected by the precipitation regime in the 2 years (2018, 2019) (Fig. 4, Supplementary Information Table 7). Plant leaf δ15N values did not respond to drought in 2018, but were significantly higher under drought in 2019. Plant δ15N values were significantly elevated in the Chernozem in comparison to the Phaeozem, with a significant decrease in plant δ15N from 2018 to 2019 (Table 3). This likely results from higher soil microbial activity, and therefore greater decomposition, mineralization, and plant uptake of the 15N-labeled green manure in the Chernozem soil and low rates of conventional N fertilization in 2018.
In summary, we suspected an initial lower turnover of green manure N (mineralization and nitrification), which is compensated over time and if wetter periods occur (heavy rainfall event, winter). However, less plant N uptake (due to a reduction of the plant biomass) and to a minor extent, lower gaseous emission losses still lead to an accumulation of green manure derived N in the soil, which is especially visible in the second year.
Conclusions
Predicted precipitation scenarios decelerated soil C and N turnover. While lower C input due to lower plant biomass production might outbalance this effect, soil N might accumulate over the years, if N fertilizer input is not reduced. Both, C and especially N, are prone to ecosystem gaseous and hydrological losses, if the fields are irrigated in the near future. Conversion of green manuring instead of synthetic fertilizer addition might help to prevent peak N losses under future irrigation regimes. Fertile soil conditions might ameliorate drought effects and delay the time point of intervention, but cannot avoid adaptation strategies. Our study emphasized the resistance and resilience of the soil C and N cycle to an altered precipitation pattern, but the impact of elevated temperature and CO2 concentrations needs to be included in future experiments to gain the full picture.
In soil biota, a drought impact was only detected in oribatida, but was not visible at a lower taxonomic resolution (bacterial and fungal PLFAs). This would underpin current knowledge that a genetically diverse community can adapt to environmental stress easier than a single species or a group with low species richness. However, due to the selection of fixed sampling dates in our experimental setup, and hence ignoring species-specific reaction patterns to drying-rewetting cycle kinetics, impacts on r-strategists (e.g., collembola) were less well represented, possibly underestimating effects on them. Following this, we conclude that the sensitivity and applicability of individual parameters to monitor the impact of drought in field situations and in the long-term vary largely, and there is still need to investigate and discuss suitable parameters to monitor the impact of climate change on soil ecosystems.
Change history
24 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00374-023-01742-4
References
Alvarez T, Frampton GK, Goulson D (1999) The effects of drought upon epigeal Collembola from arable soils. Agric For Entomol 1:243–248. https://doi.org/10.1046/j.1461-9563.1999.00032.x
APCC (2014) Austrian Assessment Report 2014 (AAR14). Verlag der Österreichischen Akademie der Wissenschaften, Wien
Aupic-Samain A, Santonja M, Chomel M, Pereira S, Quer E, Lecareux C, Limousin JM, Ourcival JM, Simioni G, Gauquelin T, Fernandez C, Baldy V (2021) Soil biota response to experimental rainfall reduction depends on the dominant tree species in mature northern Mediterranean forests. Soil Biol Biochem 154:108122. https://doi.org/10.1016/j.soilbio.2020.108122
Bardgett RD, Caruso T (2020) Soil microbial community responses to climate extremes: resistance, resilience and transitions to alternative states. Philos Trans R Soc B Biol Sci 375. https://doi.org/10.1098/rstb.2019.0112
Bardgett RD, Jones AC, Jones DL, Kemmitt SJ, Cook R, Hobbs PJ (2001) Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol Biochem 33:1653–1664. https://doi.org/10.1016/S0038-0717(01)00086-4
Bardgett RD, McAlister E (1999) The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fertil Soils 29:282–290. https://doi.org/10.1007/s003740050554
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388. https://doi.org/10.1007/s00374-005-0858-3
Behan-Pelletier VM (1999) Oribatid mite biodiversity in agroecosystems: role for bioindication. Agric Ecosyst Environ 74:411–423
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant and Soil 10:9–31
Blankinship JC, Niklaus PA, Hungate BA (2011) A meta-analysis of responses of soil biota to global change. Oecologia 165:553–565. https://doi.org/10.1007/s00442-011-1909-0
BMLFUW (2017) Richtlinie Für Die Interpretation Von Bodenuntersuchungs- Ergebnissen. Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft, In, pp 1–115
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Chang Biol 15:808–824. https://doi.org/10.1111/j.1365-2486.2008.01681.x
Brangarí AC, Manzoni S, Rousk J (2021) The mechanisms underpinning microbial resilience to drying and rewetting – a model analysis. Soil Biol Biochem 162:108400. https://doi.org/10.1016/j.soilbio.2021.108400
Canarini A, Kiær LP, Dijkstra FA (2017) Soil carbon loss regulated by drought intensity and available substrate: a meta-analysis. Soil Biol Biochem 112:90–99. https://doi.org/10.1016/j.soilbio.2017.04.020
Canarini A, Schmidt H, Fuchslueger L, Martin V, Herbold CW, Zezula D, Gündler P, Hasibeder R, Jecmenica M, Bahn M, Richter A (2021) Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat Commun 12:1–14. https://doi.org/10.1038/s41467-021-25675-4
Chahartaghi M, Langel R, Scheu S, Ruess L (2005) Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol Biochem 37:1718–1725. https://doi.org/10.1016/j.soilbio.2005.02.006
Cole L, Bradford MA, Shaw PJA, Bardgett RD (2006) The abundance, richness and functional role of soil meso- and macrofauna in temperate grassland-a case study. Appl Soil Ecol 33:186–198. https://doi.org/10.1016/j.apsoil.2005.11.003
Crocetti L, Forkel M, Fischer M, Jurečka F, Grlj A, Salentinig A, Trnka M, Anderson M, Ng WT, Kokalj Ž, Bucur A, Dorigo W (2020) Earth observation for agricultural drought monitoring in the Pannonian Basin (southeastern Europe): current state and future directions. Reg Environ Chang 20. https://doi.org/10.1007/s10113-020-01710-w
De Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811. https://doi.org/10.1016/j.femsre.2004.11.005
De Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J (2006) Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem 38:2092–2103. https://doi.org/10.1016/j.soilbio.2006.01.008
De Vries FT, Shade A (2013) Controls on soil microbial community stability under climate change. Front Microbiol 4:1–16. https://doi.org/10.3389/fmicb.2013.00265
Deng L, Peng C, Kim DG, Li J, Liu Y, Hai X, Liu Q, Huang C, Shangguan Z, Kuzyakov Y (2021) Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Science Rev 214:103501. https://doi.org/10.1016/j.earscirev.2020.103501
Djukic I, Zehetner F, Watzinger A, Horacek M, Gerzabek MH (2013) In situ carbon turnover dynamics and the role of soil microorganisms therein: a climate warming study in an Alpine ecosystem. FEMS Microbiol Ecol 83:112–124. https://doi.org/10.1111/j.1574-6941.2012.01449.x
Evans SE, Burke IC (2013) Carbon and nitrogen decoupling under an 11-year drought in the shortgrass steppe. Ecosystems 16:20–33. https://doi.org/10.1007/s10021-012-9593-4
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625. https://doi.org/10.1016/j.soilbio.2010.11.021
Gallucci M (2019) GAMLj: general analyses for linear models. jamovi module. https://gamlj.github.io/.
Gardi C, Montanarella L, Arrouays D, Bispo A, Lemanceau P, Jolivet C, Mulder C, Ranjard L, Römbke J, Rutgers M, Menta C (2009) Soil biodiversity monitoring in Europe: ongoing activities and challenges. Eur J Soil Sci 60:807–819. https://doi.org/10.1111/j.1365-2389.2009.01177.x
Gerzabek MH, Krenn A (1996) Errichtung der Lysimeteranlage Hirschstetten. In: Bundesamt für Alpenländische Landwirtschaft. Tagungsband der 6, Gumpensteiner Lysimetertagung, pp 33–36
Harris D, Horwa WR, Van KC (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon. Soil Sci Soc Am J 1856:1853–1856
Heiling M, Arrillaga J, Hood-Nowotny R, Videla X (2006) Preparation of ammonium-15N and nitrate-15N samples by microdiffusion for isotope ratio analysis by optical emission spectrometry. Commun Soil Sci Plant Anal 37:337–346. https://doi.org/10.1080/00103620500439931
Holmstrup M, Damgaard C, Schmidt IK et al (2017) Long-term and realistic global change manipulations had low impact on diversity of soil biota in temperate heathland. Sci Rep 7:1–11. https://doi.org/10.1038/srep41388
Holmstrup M, Sørensen JG, Schmidt IK, Nielsen PL, Mason S, Tietema A, Smith AR, Bataillon T, Beier C, Ehlers BK (2013) Soil microarthropods are only weakly impacted after 13 years of repeated drought treatment in wet and dry heathland soils. Soil Biol Biochem 66:110–118. https://doi.org/10.1016/j.soilbio.2013.06.023
Homyak PM, Allison SD, Huxman TE, Goulden ML, Treseder KK (2017) Effects of drought manipulation on soil nitrogen cycling: a meta-analysis. J Geophys Res Biogeo 122:3260–3272. https://doi.org/10.1002/2017JG004146
Hood-Nowotny R, Umana NH-N, Inselbacher E, Oswald- Lachouani P, Wanek W (2010) Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci Soc Am J 74:1018–1027. https://doi.org/10.2136/sssaj2009.0389
IPCC (2021) Summary for Policymakers. In: Climate Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Masson-Delmotte V, Zhai P, Pirani A, Connors S L , Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis M I, Huang M, Leitzell K, Lonnoy E, Matthews J B R , Maycock T K , Waterfield T, Yelekçi O, Yu R and Zhou B (eds.). Cambridge University Press
Jacob D, Petersen J, Eggert B et al (2014) EURO-CORDEX: new high-resolution climate change projections for European impact research. Reg Environ Chang 14:563–578. https://doi.org/10.1007/s10113-013-0499-2
Joergensen RG (2022) Phospholipid fatty acids in soil—drawbacks and future prospects. Biol Fertil Soils 58:1–6. https://doi.org/10.1007/s00374-021-01613-w
Kardol P, Reynolds WN, Norby RJ, Classen AT (2011) Climate change effects on soil microarthropod abundance and community structure. Appl Soil Ecol 47:37–44. https://doi.org/10.1016/j.apsoil.2010.11.001
Keeling C (1958) The concentration and isotopic abundance of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Acta 13:322–334
Lachouani P, Frank AH, Wanek W (2010) A suite of sensitive chemical methods to determine the δ15N of ammonium, nitrate and total dissolved N in soil extracts. Rapid Commun Mass Spectrom 24:3615–3623. https://doi.org/10.1002/rcm.4798
Leitner S, Hood-Nowotny R, Watzinger A (2020) Successive and automated stable isotope analysis of CO2, CH4 and N2O paving the way for unmanned aerial vehicle-based sampling. Rapid Commun Mass Spectrom 34. https://doi.org/10.1002/rcm.8929
Leizeaga A, Meisner A, Rousk J, Bååth E (2022) Repeated drying and rewetting cycles accelerate bacterial growth recovery after rewetting. Biol Fertil Soils 58:365–374. https://doi.org/10.1007/s00374-022-01623-2
Lennon JT, Aanderud ZT, Lehmkuhl BK, Schoolmaster DR (2012) Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology 93:1867–1879. https://doi.org/10.1890/11-1745.1
Leuprecht A, Chimani B, Hofstätter M, Kerschbaumer M, Kienberger S, Kottek M, Truhetz H, Lexer A, Peßenteiner S (2016) ÖKS15 bias corrected EURO-CORDEX model precipitation: CCCA Data Centre. https://hdl.handle.net/20.500.11756/f6748f11
Li L, Zheng Z, Wang W, Biederman JA, Xu X, Ran Q, Qian R, Xu C, Zhang B, Wang F, Zhou S, Cui L, Che R, Hao Y, Cui X, Xu Z, Wang Y (2020) Terrestrial N2O emissions and related functional genes under climate change: a global meta-analysis. Glob Chang Biol 26:931–943. https://doi.org/10.1111/gcb.14847
Lindberg N, Bengtsson J (2006) Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos 114:494–506. https://doi.org/10.1111/j.2006.0030-1299.14396.x
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93:930–938. https://doi.org/10.1890/11-0026.1
Meyer S, Kundel D, Birkhofer K, Fliessbach A, Scheu S (2021) Soil microarthropods respond differently to simulated drought in organic and conventional farming systems. Ecol Evol 11:10369–10380. https://doi.org/10.1002/ece3.7839
Naylor D, Coleman-Derr D (2018) Drought stress and root-associated bacterial communities. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.02223
Peguero G, Folch E, Liu L, Ogaya R, Peñuelas J (2021) Divergent effects of drought and nitrogen deposition on microbial and arthropod soil communities in a Mediterranean forest. Eur J Soil Biol 103:103275. https://doi.org/10.1016/j.ejsobi.2020.103275
Pollierer MM, Scheu S (2017) Driving factors and temporal fluctuation of Collembola communities and reproductive mode across forest types and regions. Ecol Evol 7:4390–4403. https://doi.org/10.1002/ece3.3035
Postma-Blaauw MB, De Goede RGM, Bloem J, Faber JH, Brussaard L (2010) Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 91:460–473. https://doi.org/10.1890/09-0666.1
Preece C, Verbruggen E, Liu L, Weedon JT, Peñuelas J (2019) Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biol Biochem 131:28–39. https://doi.org/10.1016/j.soilbio.2018.12.022
Pugnaire FI, Morillo JA, Peñuelas J, Reich PB, Bardgett RD, Gaxiola A, Wardle DA, Van Der Putten WH (2019) Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci Adv 5:1–12. https://doi.org/10.1126/sciadv.aaz1834
R Core Team (2020) R: a language and environment for statistical computing. (Version 4.0). https://cran.r-project.org
Ren C, Zhao F, Shi Z, Chen J, Han X, Yang G, Feng Y, Ren G (2017) Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol Biochem 115:1–10. https://doi.org/10.1016/j.soilbio.2017.08.002
Rousk J, Bååth E (2007) Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol Ecol 62:258–267. https://doi.org/10.1111/j.1574-6941.2007.00398.x
Sackett TE, Classen AT, Sanders NJ (2010) Linking soil food web structure to above- and belowground ecosystem processes: a meta-analysis. Oikos 119:1984–1992. https://doi.org/10.1111/j.1600-0706.2010.18728.x
Santonja M, Fernandez C, Proffit M, Gers C, Gauquelin T, Reiter IM, Cramer W, Baldy V (2017) Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest. J Ecol 105:801–815. https://doi.org/10.1111/1365-2745.12711
Schimel JP (2018) Life in dry soils: effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst 49:409–432. https://doi.org/10.1146/annurev-ecolsys-110617-062614
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00348
Seastedt TR (1984) The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol 29:25–46. https://doi.org/10.1146/annurev.en.29.010184.000325
Seeber J, Rief A, Richter A, Traugott M, Bahn M (2012) Drought-induced reduction in uptake of recently photosynthesized carbon by springtails and mites in alpine grassland. Soil Biol Biochem 55:37–39. https://doi.org/10.1016/j.soilbio.2012.06.009
Seidl R, Albrich K, Erb K, Formayer H, Leidinger D, Leitinger G, Tappeiner U, Tasser E, Rammer W (2019) What drives the future supply of regulating ecosystem services in a mountain forest landscape? For Ecol Manage 445:37–47. https://doi.org/10.1016/j.foreco.2019.03.047
Setia R, Verma SL, Marschner P (2012) Measuring microbial biomass carbon by direct extraction - comparison with chloroform fumigation-extraction. Eur J Soil Biol 53:103–106. https://doi.org/10.1016/j.ejsobi.2012.09.005
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205. https://doi.org/10.1016/j.soilbio.2019.01.010
Stark JM, Firestone MK (1995) Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61:218–221. https://doi.org/10.1128/aem.61.1.218-221.1995
Stemmer M, Watzinger A, Blochberger K, Haberhauer G, Gerzabek MH (2007) Linking dynamics of soil microbial phospholipid fatty acids to carbon mineralization in a 13C natural abundance experiment: impact of heavy metals and acid rain. Soil Biol Biochem 39:3177–3186
Stenitzer E, Hösch J (2005) Grundwasserneubildung im Marchfeld – Lysimetermessungen und Modellrechnungen. In: Bundesamt für Alpenländische Landwirtschaft. Tagungsband der 11, Gumpensteiner Lysimetertagung, pp 41–44
Strickland MS, Rousk J (2010) Considering fungal: bacterial dominance in soils - methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. https://doi.org/10.1016/j.soilbio.2010.05.007
Switanek BM, Troch AP, Castro LC, Leuprecht A, Chang HI, Mukherjee R, Demaria MCE (2017) Scaled distribution mapping: a bias correction method that preserves raw climate model projected changes. Hydrol Earth Syst Sci 21:2649–2666. https://doi.org/10.5194/hess-21-2649-2017
Tataw JT, Baier F, Krottenthaler F, Pachler B, Schwaiger E, Wyhlidal S, Formayer H, Hösch J, Baumgarten A, Zaller JG (2016) Climate change induced rainfall patterns affect wheat productivity and agroecosystem functioning dependent on soil types. Ecol Res 31:203–212. https://doi.org/10.1007/s11284-015-1328-5
Tataw JT, Hall R, Ziss E, Schwarz T, von Hohberg und Buchwald C, Formayer H, Hösch J, Baumgarten A, Berthold H, Michel K, Zaller JG (2014) Soil types will alter the response of arable agroecosystems to future rainfall patterns. Ann Appl Biol 164:35–45. https://doi.org/10.1111/aab.12072
Thaler S, Formayer H, Kubu G, Trnka M, Eitzinger J (2021) Effects of bias-corrected regional climate projections and their spatial resolutions on crop model results under different climatic and soil conditions in Austria. Agric 11:1–39. https://doi.org/10.3390/agriculture11111029
The jamovi project (2021) jamovi. (Version 1.6). https://www.jamovi.org
Tsiafouli MA, Thébault E, Sgardelis SP et al (2015) Intensive agriculture reduces soil biodiversity across Europe. Glob Chang Biol 21:973–985. https://doi.org/10.1111/gcb.12752
van Meeteren MJM, Tietema A, van Loon EE, Verstraten JM (2008) Microbial dynamics and litter decomposition under a changed climate in a Dutch heathland. Appl Soil Ecol 38:119–127. https://doi.org/10.1016/j.apsoil.2007.09.006
Vestergård AM, Dyrnum K, Michelsen A, Damgaard C, Holmstrup M (2015) Long-term multifactorial climate change impacts on mesofaunal biomass and nitrogen content. Appl Soil Ecol 92:54–63. https://doi.org/10.1016/j.apsoil.2015.03.002
Wang P, Zhou W, Niu Z, Xiong X, Wu S, Cheng P, Hou Y, Lu X, Du H (2021) Spatio-temporal variability of atmospheric CO2 and its main causes: a case study in Xi’an city. China Atmos Res 249:105346. https://doi.org/10.1016/j.atmosres.2020.105346
Watzinger A (2015) Microbial phospholipid biomarkers and stable isotope methods help reveal soil functions. Soil Biol Biochem 86:98–107. https://doi.org/10.1016/j.soilbio.2015.03.019
Watzinger A, Hood-Nowotny R (2019) Stable isotope probing of microbial phospholipid fatty acids in environmental samples. In: Dumont MG, García MH (eds) Stable Isotope Probing. Humana Press, USA, pp 45–55
Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Chang Biol 17:927–942. https://doi.org/10.1111/j.1365-2486.2010.02302.x
Xu GL, Kuster TM, Günthardt-Goerg MS, Dobbertin M, Li MH (2012) Seasonal exposure to drought and air warming affects soil collembola and mites. PloS One 7:23–27. https://doi.org/10.1371/journal.pone.0043102
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129. https://doi.org/10.1007/s003740050533
Acknowledgements
Alexander Bruckner, Institute of Zoology, BOKU University of Natural Resources and Life Sciences Vienna for mesofauna extraction and determination.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The work was funded by the Austrian Climate ResearA.ch Programme 9 under the project name Climagrocycle (KR16AC0K13275).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to the spelling correction of the co-author's family name.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watzinger, A., Prommer, J., Spiridon, A. et al. Functional redundant soil fauna and microbial groups and processes were fairly resistant to drought in an agroecosystem. Biol Fertil Soils 59, 629–641 (2023). https://doi.org/10.1007/s00374-023-01728-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01728-2