Abstract
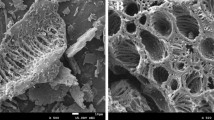
Cadmium is both readily available and highly toxic to plants and animals. Our objectives were to evaluate the effect of oyster shell as a liming material on reducing plant cadmium (Cd) uptake and to compare oyster shell and Ca(OH)2, a common liming material in Korea. Ground oyster shell and Ca(OH)2 were applied at 0, 2, 4, and 8 Mg Ca per hectare to an upland soil contaminated manually with CdSO4 (total Cd 8.96 mg kg−1). Radish (Raphanus sativa L.) was sown on the contaminated soil. Oyster shell was less effective at increasing soil pH and net negative charge than Ca(OH)2, but more effective at suppressing radish Cd uptake in both roots and shoots. The portion of Cd that is strongly bound to soil (fraction 5) increased more with oyster shell than with Ca(OH)2. Radish plant Cd concentration was positively correlated with 0.1 N HCl-extractable Cd and negatively correlated with the residual Cd fraction (F5), indicating that an increase in the strongly bound Cd fraction played an important role in reducing radish Cd uptake in soil to which oyster shell was applied. The greater potential of oyster shell to decrease Cd extractability in soil and plant Cd uptake compared to Ca(OH)2 might be attributed to the layered morphology of oyster shells. Based on these results, oyster shell could be a very good alternative liming material to reduce Cd phytoavailability in Cd-contaminated soil.




Similar content being viewed by others
References
Adriano DC (2001) Trace elements in terrestrial environments; biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York, p 866
Allison LE (1965) Organic carbon. In: Black CA (ed) Methods of soil analysis, Part II. American Society of Agronomy Inc., Madison, pp 1367–1378
Barrow NJ (1985) Reactions of anions and cations with variable charge soils. Adv Agron 38:183–230
Bingham FT, Page AL, Mitchell GA, Strong JE (1979) Effects of liming an acid soil amended with sewage sludge enriched with Cd, Cu, Ni, and Zn on yield and Cd content of wheat-grain. J Environ Qual 8:202–207
Bolan NS, Naidu R, Syers JK, Tillman RW (1999) Surface charge and solute interactions in soils. Adv Agron 67:88–141
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251:187–198
Brallier S, Harrison RB, Henry CL, Dongsen X (1996) Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Water Air Soil Pollut 86:195–206
Chlopecka A, Adriano DC (1996) Mimicked in-situ stabilization of metals in a cropped soil: bioavailability and chemical form of zinc. Environ Sci Technol 30:3294–3303
Curtin D, Campbell CA, Messer D (1996) Prediction of titratable acidity and soil sensitivity to pH change. J Environ Qual 25:1280–1284
Fernandes ML, Abreu MM, Calouro F, Vaz MC (1999) Effect of liming and cadmium application in an acid soil on cadmium availability to Sudan grass. Commun Soil Sci Plant Anal 30:1051–1062
Hong CO, Lee DK, Chung DY, Kim PJ (2007) Liming effects on cadmium stabilization in upland soil affected by gold mining activity. Arch Environ Contam Toxicol 52:492–502
Hong CO, Lee DK, Kim PJ (2008) Feasibility of phosphate fertilizer to immobilize cadmium in a field. Chemosphere 70:2009–2015
Hong CO, Gutierrez MJ, Yun SW, Lee YB, Yu C, Kim PJ (2009) Heavy metal contamination of arable soil and corn plant in the vicinity of zinc smelting factory and stabilization by liming. Arch Environ Contam Toxicol 56:190–200
John MK, VanLaerhoven CJ, Chuah HH (1972) Factors affecting plant uptake and phytotoxicity of cadmium added to soils. Environ Sci Technol 6:1005–1009
Khan DH, Frankland B (1983) Effects of cadmium and lead on radish plants with particular reference to movement of metals through soil profile and plant. Plant Soil 70:335–345
Knox AS, Seaman JC, Mench MJ, Vangronsveld J (2001) Remediation of metal- and radionuclides-contaminated soils by in situ stabilization techniques. In: Iskandar IK (ed) Environmental restoration of metals-contaminated soils, edn. CRC, Boca Raton, pp 21–60
Kwon HB, Lee CW, Jun BS, Yun JD, Weon SY, Koopman B (2004) Recycling waste oyster shells for eutrophication control. Resour Conser Recy 41:75–82
Lee CH, Lee DK, Ali MA, Kim PJ (2008) Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Manag 28:2702–2708
Lehoczky E, Marth P, Szabados I, Szomolanyi A (2000) The cadmium uptake by lettuce on contaminated soils as influenced by liming. Commun Soil Sci Plant Anal 31:2433–2438
Maclean AJ (1976) Cadmium in different plant species and its availability in soils as influenced by organic-matter and additions of lime, P, Cd and Zn. Can J Soil Sci 56:129–138
Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur J Soil Sci 45:419–459
RDA (Rural Development Administration, Korea) (1988) Methods of soil chemical analysis. National Institute of Agricultural Science and Technology, RDA. Suwon (in Korean)
SAS Institute Inc (1996) SAS system for mixed models. SAS Institute, Cary
SAS Institute Inc (2001) User’s guide: statistics SAS version 8.2. SAS Institute, Cary
Schofield RK (1949) Effect of pH on elentric changes carried by clay particles. J Soil Sci 1:1–8
Singh BR, Narwal RP, Jeng AS, Almas A (1995) Crop uptake and extractability of cadmium in soils naturally high in metals at different pH levels. Commun Soil Sci Plant Anal 26:2123–2142
Sposito F, Sund LJ, Chang AC (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd and Pb in solid phases. Soil Sci Soc Am J 46:260–264
Street JJ, Lindsay WL, Sabey BR (1977) Solubility and plant uptake of cadmium in soils amended with cadmium and sewage sludge. J Environ Qual 6:72–77
Thomas GW, Hargrove WL (1984) The Chemistry of soil acidity. In: Adams F (ed) Soil acidity and liming, 2nd edn. Agron Monogr 12. American Society of Agronomy, Madison, pp 3–56
Tyler G, Olsson T (2001) Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant Soil 230:307–321
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. In J Environ Anal Chem 51:135–151
Weast RC, Astle MJ (1978–1979) Handbook of chemistry and physics, 5th edn. CRC, Boca Raton
Xian X (1989) Effect of chemical forms of cadmium, zinc and lead in polluted soils on their uptake by cabbage plants. Plant Soil 113:257–264
Acknowledgments
Sang Yoon Kim was supported by scholarships from the BK21 Program, Ministry of Education & Resources Development, Korea. This study was carried out with the support of “On-Site Cooperative Agriculture Research Project (20070401-080-100-001-01-00)”, RDA, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, C.O., Kim, S.Y., Gutierrez, J. et al. Comparison of oyster shell and calcium hydroxide as liming materials for immobilizing cadmium in upland soil. Biol Fertil Soils 46, 491–498 (2010). https://doi.org/10.1007/s00374-010-0458-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0458-8