Abstract
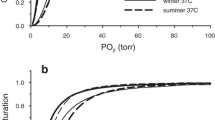
The Asiatic toad (Bufo gargarizans) belonging to the family of Bufonidae (Anura: Amphibia) is successfully residing on the Qinghai–Tibetan Plateau (QTP). To investigate whether the oxygen delivery undergoes adaptive adjustments to high-altitude environments in Asian toads inhabiting the QTP (Zoige County, 3446 m), choosing low-altitude populations (Chengdu City, 500 m) as control, we measured hematological traits, O2 affinities of whole blood, Hb-O2 affinities of purified Hbs, their sensitivities to temperature, and allosteric effectors (H+, Cl− and ATP). Our results showed that high-altitude Asiatic toads possessed significantly increased hemoglobin concentration, hematocrit, and red blood cell count, but significantly decreased erythrocyte volume compared with low-altitude toads. The whole blood and purified Hbs of high-altitude Asiatic toads both exhibited significantly higher O2 affinities compared with low-altitude toads. Substantially increased intrinsic Hb-O2 affinities of high-altitude Asiatic toads Hbs are likely to be the main reason for its elevated Hb-O2 affinities given the anionic cofactor sensitivities of high- and low-altitude toads were similar. The Hbs of high-altitude toads were also characterized by distinctly strong Bohr effects at the low temperature and low-temperature sensitivities. The adaptive adjustments of hematological traits could enhance the blood–O2 carrying capacity of high-altitude Asiatic toads. The increased Hb-O2 affinities could safeguard the pulmonary O2 uploading under hypoxia. The strong Bohr effects at the low temperature could help the release of O2 in metabolic tissues and cold limbs, while low-temperature sensitivity could minimize the effect of temperature fluctuation on the Hb–O2 affinity.



Similar content being viewed by others
Abbreviations
- Hbs:
-
Hemoglobins
- QTP:
-
Qinghai–Tibetan Plateau
- DPG:
-
2,3-Diphosphoglycerate
- IP5:
-
Inositol pentaphosphate
- ATP:
-
Adenosine triphosphate
- [Hb]:
-
Hemoglobin concentration
- RBC:
-
Red blood cell
- Hct:
-
Hematocrit
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCH:
-
Mean cell hemoglobin
- MCV:
-
Mean corpuscular volume
References
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. North-Holland Publishing Company, Amsterdam
Bartlett GR (1959) Colorimetric assay methods for free and phosphorylated glyceric acids. J Biol Chem 234:469–471
Beall CM (2007) Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci 104:8655–8660
Bonaventura C, Ferruzzi G, Tesh S, Stevens RD (1999) Effects of S-nitrosation on oxygen binding by normal and sickle cell hemoglobin. J Biol Chem 274:24742–24748
Brix O, Bårdgard A, Mathisen S, Tyler N, Nuutinen M, Condo SG, Giardina B (1990) Oxygen transport in the blood of arctic mammals: adaptation to local heterothermia. J Comp Physiol B 159:655–660
Cabagna MC, Lajmanovich RC, Stringhini G, Sanchez-Hernandez JC, Peltzer PM (2005) Hematological parameters of health status in the common toad Bufo arenarum in agroecosystems of Santa Fe Province. Argentina Appl Herpetol 2:373
Carmena-Suero A, Siret JR, Caixejas J, Arpones-Carmena D (1980) Blood volume in male Hyla septentrionalis (tree frog) and Rana catesbeiana (bullfrog). Comp Biochem Physiol A Physiol 67:187–189
Coletta M, Clementi ME, Ascenzi P, Petruzzelli R, Condò SG, Giardina B (1992) A comparative study of the temperature dependence of the oxygen-binding properties of mammalian hemoglobins. Eur J Biochem 204:1155–1157
Connes P, Yalcin O, Baskurt O, Brun J-F, Hardeman M (2006) In health and in a normoxic environment, VO2 max is/is not limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol 100:2099–2099
Damsgaard C, Storz JF, Hoffmann FG, Fago A (2013) Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am J Physiol Regulat Integr Comp Physiol 305:R961–R967
Dönmez F, Tosunoğlu M, Gül Ç (2009) Hematological values in hermaphrodite, Bufo bufo (Linnaeus, 1758). North-Western J Zool 5:97–103
Dunlap KD (2006) Ontogeny and scaling of hematocrit and blood viscosity in western fence lizards, Sceloporus occidentalis. Copeia 2006:535–538
Favre A, Päckert M, Pauls SU, Jähnig SC, Uhl D, Michalak I, Muellner-Riehl AN (2015) The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol Rev 90:236–253
Fei L, Hu S, Ye C, Huang Y (2009) Fauna sinica, amphibia, vol 2. Science Press, Beijing
Guyton AC, Richardson TQ (1961) Effect of hematocrit on venous return. Circ Res 9:157–164
Hazard ES, Hutchison VH (1982) Distribution of acid-soluble phosphates in the erythrocytes of selected species of amphibians. Comp Biochem Physiol A Physiol 73:111–124
Hillman SS (1980) Physiological correlates of differential dehydration tolerance in anuran amphibians. Copeia 1980:125–129
Hutchison VH, Haines HB, Engbretson G (1976) Aquatic life at high altitude: respiratory adaptations in the Lake Titicaca frog, Telmatobius culeus. Respir Physiol 27:115–129
Jendroszek A et al (2018) Allosteric mechanisms underlying the adaptive increase in hemoglobin–oxygen affinity of the bar-headed goose. J Exp Biol 221:jeb185470
Jensen B, Storz JF, Fago A (2016) Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp Biochem Physiol A Mol Integr Physiol 195:10–14
Johansen K, Lykkeboe G, Kornerup S, Maloiy GMO (1980) Temperature insensitive O2 in blood of the tree frog Chiromantis petersi. J Comp Physiol 136:71–76
Kumar A, Natarajan C, Moriyama H, Witt CC, Weber RE, Fago A, Storz JF (2017) Stability-mediated epistasis restricts accessible mutational pathways in the functional evolution of avian hemoglobin. Mol Biol Evol 34:1240–1251
Liao W, Lu X (2012) Adult body size = f (initial size + growth rate × age): explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol Ecol 26:579–590
Lorenzo FR et al (2014) A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46:951–956
Lu S et al (2015) Differences in hematological traits between high- and low-altitude lizards (Genus Phrynocephalus). PLoS ONE 10:e0125751
McClelland GB, Scott GR (2019) Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu Rev Physiol 81:561–583
Moharram N et al (2006) International collaborative assessment study of the AHD [575] method for the measurement of blood haemoglobin. East Mediterr Health J 12:722
Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF (2013) Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340:1324–1327
Natarajan C et al (2015) Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet 11:e1005681
Natarajan C, Jendroszek A, Kumar A, Weber RE, Tame JRH, Fago A, Storz JF (2018) Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet 14:e1007331
Ostojic H, Monge-C C, Cifuentes V (2000) Hemoglobin affinity for oxygen in three subspecies of toads (Bufo sp.) living at different altitudes. Biol Res 33:5–10
Pinder A, Burggren W (1983) Respiration during chronic hypoxia and hyperoxia in larval and adult bullfrogs (Rana catesbeiana). II. Changes in respiratory properties of whole blood. J Exp Biol 105:205–213
Pu P et al (2019) Oxygenation properties and underlying molecular mechanisms of hemoglobins in plateau zokor (Eospalax baileyi). Am J Physiol Regulat Integr Comp Physiol 317:R696–R708
Qiu Q et al (2012) The yak genome and adaptation to life at high altitude. Nat Genet 44:946–949
Qu Y et al (2013) Ground tit genome reveals avian adaptation to living at high altitudes in the Tibetan plateau. Nat Commun 4:2071
Rollema HS, Bauer C (1979) The interaction of inositol pentaphosphate with the hemoglobins of highland and lowland geese. J Biol Chem 254:12038
Ruiz G, Rosenmann M, Veloso A (1983) Respiratory and hematological adaptations to high altitude in Telmatobius frogs from the Chilean Andes. Comp Biochem Physiol A Physiol 76:109–113
Simonson TS et al (2010) Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75
Storz JF (2016) Hemoglobin–oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J Exp Biol 219:3190–3203
Storz JF, Moriyama H (2008) Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt Med Biol 9:148–157
Storz JF et al (2009) Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci 106:14450–14455
Storz JF, Runck AM, Moriyama H, Weber RE, Fago A (2010a) Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol 213:2565–2574
Storz JF, Scott GR, Cheviron ZA (2010b) Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol 213:4125–4136
Tufts DM et al (2014) Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol Biol Evol 32:287–298
Wang H et al (2018) Function of lactate dehydrogenase in cardiac and skeletal muscle of Phrynocephalus lizard in relation to high-altitude adaptation. Asian Herpetol Res 9:258–273
Weber RE (1981) Cationic control of O2 affinity in lugworm erythrocruorin. Nature 292:386–387
Weber RE (1992) Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol 72:1611–1615
Weber RE, Campbell KL (2011) Temperature dependence of haemoglobin–oxygen affinity in heterothermic vertebrates: mechanisms and biological significance. Acta Physiol 202:549–562
Weber RE, Jessen TH, Malte H, Tame J (1993) Mutant hemoglobins (alpha 119-Ala and beta 55-Ser): functions related to high-altitude respiration in geese. J Appl Physiol 75:2646–2655
Weber RE, Ostojic H, Fago A, Dewilde S, Hauwaert M-LV, Moens L, Monge C (2002) Novel mechanism for high-altitude adaptation in hemoglobin of the Andean frog Telmatobius peruvianus. Am J Physiol Regulat Integr Comp Physiol 283:R1052–R1060
Weber RE, Fago A, Campbell KL (2014) Enthalpic partitioning of the reduced temperature sensitivity of O2 binding in bovine hemoglobin. Comp Biochem Physiol A Mol Integr Physiol 176:20–25
Weber RE, Jarvis JUM, Fago A, Bennett NC (2017) O2 binding and CO2 sensitivity in hemoglobins of subterranean African mole rats. J Exp Biol 220:3939–3948
Wen G, Yang W, Fu J (2015) Population genetic structure and species status of asiatic toads (Bufo gargarizans) in Western China. Zoolog Sci 32(427–434):428
Wood SC, Johansen K (1972) Adaptation to hypoxia by increased HbO2 affinity and decreased red cell ATP concentration. Nat New Biol 237:278–279
Xiong J et al (2018) Comparison of hematological parameters in two different high altitudinal populations of Batrachuperus pinchonii (Amphibian: Urodela). Amphibia-Reptilia 39:11–20
Yang W, Qi Y, Fu J (2016) Genetic signals of high-altitude adaptation in amphibians: a comparative transcriptome analysis. BMC Genet 17:134
Yang W, Qi Y, Lu B, Qiao L, Wu Y, Fu J (2017) Gene expression variations in high-altitude adaptation: a case study of the Asiatic toad (Bufo gargarizans). BMC Genet 18:62
Zhan A, Fu J (2011) Past and present: phylogeography of the Bufo gargarizans species complex inferred from multi-loci allele sequence and frequency data. Mol Phylogenet Evol 61:136–148
Zhou Y, Liu G, Wen D (1984) A preliminary study on the red cell 2, 3-diphosphoglycerate in the pika and the zokor. Acta Biol Plateau Sinica 2:133–137
Zhu X et al (2018) Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. Proc Natl Acad Sci 115:1865–1870
Acknowledgements
We thank Yaofeng Zhao for his assistance in the acquisition of experimental animals. We thank the Core Facility of School of Life Sciences, Lanzhou University, for technical assistance.
Funding
This work was funded by the National Natural Science Foundation of China (No. 31971416 to Qiang Chen) and the Fundamental Research Funds for the Central Universities (lzujbky-2019-it16 to Peng Pu).
Author information
Authors and Affiliations
Contributions
Conceptualization: QC and PP; methodology: PP, YZ, and ZN; software: PP, YZ, and JH; formal analysis: PP, JW, WC, and TZ; investigation: PP, YZ, and TZ; resources: QC, XT, and PP; data curation: QC, YZ, and PP; writing—original draft: PP and QC; writing review and editing: QC, PP, XT, YZ, ZN, JH, WC, and TZ; visualization: PP, ZN, and JW; supervision: QC and PP; project administration: QC; funding acquisition: QC and PP.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by E. Polymeropoulos.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pu, P., Zhao, Y., Niu, Z. et al. Comparison of hematological traits and oxygenation properties of hemoglobins from highland and lowland Asiatic toad (Bufo gargarizans). J Comp Physiol B 191, 1019–1029 (2021). https://doi.org/10.1007/s00360-021-01368-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-021-01368-8