Abstract
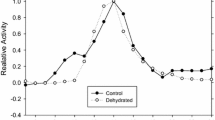
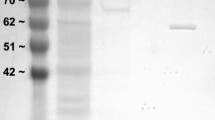
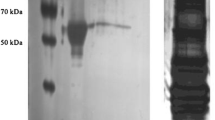
The African clawed frog, Xenopus laevis endures whole body dehydration which can increase its reliance on anaerobic glycolysis for energy production. This makes the regulation of the terminal enzyme of glycolysis, lactate dehydrogenase (LDH), crucial to stress survival. We investigated the enzymatic properties and posttranslational modification state of purified LDH from the skeletal muscle of control and dehydrated (30% total body water loss) X. laevis. LDH from the muscle of dehydrated frogs showed a 93% reduction in phosphorylation on threonine residues and an 80% reduction of protein nitrosylation. LDH from dehydrated muscle also showed a 74% lower Vmax in the pyruvate oxidizing direction and a 78% decrease in Vmax in the lactate reducing direction along with a 33% lower Km for pyruvate and a 40% higher Km for lactate. In the presence of higher levels of urea and molecular crowding by polyethylene glycol, used to mimic conditions in the cells of dehydrated animals, the Km values of control and dehydrated LDH demonstrated opposite responses. In the pyruvate oxidizing direction, control muscle LDH was unaffected by these additives, whereas the affinity for pyruvate dropped further for LDH from dehydrated muscle. The opposite effect was more pronounced in the lactate reducing direction as control LDH showed an increased affinity for lactate, whereas LDH from dehydrated animals showed a further reduction in affinity. The physiological consequences of dehydration-induced LDH regulation appear to poise the enzyme towards lactate production when urea levels are high and lactate catabolism when urea levels are low, perhaps helping to maintain glycolysis under dehydrating conditions whilst providing for the ability to recycle lactate upon rehydration.





Similar content being viewed by others
References
Abboud J, Storey KB (2013) Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ 1:e12. https://doi.org/10.7717/peerj.12
Alexander SS, Bellerby CW (1938) Experimental studies on the sexual cycle of the South African clawed toad (Xenopus laevis). I. J Exp Biol 15:74–81
Balinsky JB, Choritz EL, Coe CG, van der Schans GS (1967a) Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol 22:59–68
Balinsky JB, Choritz EL, Coe CGL, van der Schans GS (1967b) Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol 22:59–68. https://doi.org/10.1016/0010-406X(67)90166-1
Bellerby CW (1938) Experimental studies on the sexual cycle of the South African Clawed Toad (Xenopus laevis). II. J exp Biol London 15:82–90
Biggar K, Dawson N, Storey K (2012) Real-time protein unfolding: a method for determining the kinetics of native protein denaturation using a quantitative real-time thermocycler. Biotechniques 53:231–238. https://doi.org/10.2144/0000113922
Brooks SP (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13(6):906–911
Childers CL, Storey KB (2016) Post-translational regulation of hexokinase function and protein stability in the aestivating frog Xenopus laevis. Protein J 35:61–71. https://doi.org/10.1007/s10930-016-9647-0
Cohen P (2002) The origins of protein phosphorylation. Nat Cell Biol 4:E127–E130. https://doi.org/10.1038/ncb0502-e127
Dawson NJ, Bell RAV, Storey KB (2013) Purification and properties of white muscle lactate dehydrogenase from the anoxia-tolerant turtle, the red-eared slider, Trachemys scripta elegans. Enzyme Res 2013:784973. https://doi.org/10.1155/2013/784973
Eggert C, Fouquet A (2006) A preliminary biotelemetric study of a feral invasive Xenopus laevis population in France. Alytes 23:144–149
Flanigan JE, WIthers PC, Guppy M (1991) In vitro metabolic depression of tissues from the aestivating frog Neobatrachus pelobatoides. J Exp Biol 161
Gatten RE (1987) Activity metabolism of anuran amphibians: tolerance to dehydration. Physiol Zool 60:576–585. https://doi.org/10.1086/physzool.60.5.30156131
Guppy M, Withers P (1999) Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev 74:1–40
Hillman SS (1978) The roles of oxygen delivery and electrolyte levels in the dehydrational death of Xenopus laevis. J Comp Physiol B 128:169–175. https://doi.org/10.1007/BF00689481
Hillman SS, Sommerfeldt RW (1981) Microsphere studies of amphibian systemic blood flow redistribution during dehydration, hypovolemia, and salt load. J Exp Zool 218:305–308. https://doi.org/10.1002/jez.1402180223
Hochachka PW, Somero GN (1984) Biochemical adaptation. Princeton University Press, Princeton
Katzenback BA, Dawson NJ, Storey KB (2014) Purification and characterization of a urea sensitive lactate dehydrogenase from the liver of the African clawed frog, Xenopus laevis. J Comp Physiol B 184:601–611. https://doi.org/10.1007/s00360-014-0824-1
MacDonald JA, Storey KB (1999) Regulation of ground squirrel Na+K+-ATPase activity by reversible phosphorylation during hibernation. Biochem Biophys Res Commun 254:424–429. https://doi.org/10.1006/BBRC.1998.9960
MacLean IA, Mattice AMS, Adam NJ, Storey KB (2016) Purification and characterization of lactate dehydrogenase in the foot muscle and hepatopancreas of Otala lactea. Protein J 35:467–480. https://doi.org/10.1007/s10930-016-9689-3
Malik AI, Storey KB (2009) Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis. J Exp Biol 212:2595–2603. https://doi.org/10.1242/jeb.030627
Mashino T, Fridovich I (1987) Effects of urea and trimethylamine-N-oxide on enzyme activity and stability. Arch Biochem Biophys 258:356–360. https://doi.org/10.1016/0003-9861(87)90355-9
Measey G, Tinsley R (1998) Feral Xenopus laevis in south Wales. Herpetol J 8:23–28
Muir TJ, Costanzo JP, Lee RE (2007) Osmotic and metabolic responses to dehydration and urea-loading in a dormant, terrestrially hibernating frog. J Comp Physiol B Biochem Syst Environ Physiol. https://doi.org/10.1007/s00360-007-0190-3
Müller MM (2018) Post-translational modifications of protein backbones: unique functions, mechanisms, and challenges. Biochemistry 57:177–185. https://doi.org/10.1021/acs.biochem.7b00861
Oliveira AP, Sauer U (2012) The importance of post-translational modifications in regulating Saccharomyces cerevisiae metabolism. FEMS Yeast Res 12:104–117. https://doi.org/10.1111/j.1567-1364.2011.00765.x
Pace CN (1986) Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol 131:266–280. https://doi.org/10.1016/0076-6879(86)31045-0
Shahriari A, Dawson NJ, Bell RAV, Storey KB (2013) Stable suppression of lactate dehydrogenase activity during anoxia in the foot muscle of Littorina littorea and the potential role of acetylation as a novel posttranslational regulatory mechanism. Enzyme Res 2013:461374. https://doi.org/10.1155/2013/461374
Storey KB (2016) Comparative enzymology—new insights from studies of an “old” enzyme, lactate dehydrogenase. Comp Biochem Physiol Part B Biochem Mol Biol 199:13–20. https://doi.org/10.1016/J.CBPB.2015.12.004
Storey KB, Storey JM (1990) Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q Rev Biol 65:145–174
Storey KB, Storey JM (2005) Oxygen limitation and metabolic rate depression. In: Storey KB (ed) Functional metabolism. Wiley, Hoboken, pp 415–442
Storey KB, Storey JM (2012) Aestivation: signaling and hypometabolism. J Exp Biol 215:1425–1433. https://doi.org/10.1242/jeb.054403
Talaiezadeh A, Shahriari A, Tabandeh MR et al (2015) Kinetic characterization of lactate dehydrogenase in normal and malignant human breast tissues. Cancer Cell Int 15:19. https://doi.org/10.1186/s12935-015-0171-7
Uchiyama M, Konno N (2006) Hormonal regulation of ion and water transport in anuran amphibians. Gen Comp Endocrinol 147:54–61. https://doi.org/10.1016/J.YGCEN.2005.12.018
Walsh CT, Garneau-Tsodikova S, Gatto GJ (2005) Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed 44:7342–7372. https://doi.org/10.1002/anie.200501023
Xiong ZJ, Storey KB (2012) Regulation of liver lactate dehydrogenase by reversible phosphorylation in response to anoxia in a freshwater turtle. Comp Biochem Physiol Part B Biochem Mol Biol 163:221–228. https://doi.org/10.1016/J.CBPB.2012.06.001
Acknowledgements
We thank J. M. Storey for critical commentary on the manuscript. This work was supported by a Discovery Grant (#6793) from the Natural Sciences and Engineering Research Council of Canada; KBS holds the Canada Research Chair in Molecular Physiology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Childers, C.L., Storey, K.B. Purification and characterization of a urea sensitive lactate dehydrogenase from skeletal muscle of the African clawed frog, Xenopus laevis. J Comp Physiol B 189, 271–281 (2019). https://doi.org/10.1007/s00360-018-1200-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1200-3